Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Protein factors increasing yield of a biofuel precursor in microscopic algae
Press release
LIPID REMODELING REGULATOR 1 (LRL1) is differently involved in the phosphorus-depletion response from PSR1 in Chlamydomonas reinhardtii
Nur Akmalia Hidayati, Yui Yamada-Oshima, Iwai Masako, Takashi Yamano, Masataka Kajikawa, Nozomu Sakurai, Kunihiro Suda, Kanami Sesoko, Koichi Hori, Takeshi Obayashi, Mie Shimojima, Hideya Fukuzawa, Hiroyuki Ohta
The Plant Journal 27 July 2019 DOI:10.1111/tpj.14473
EurekAlert! link about this artcle
As an alternative to traditional fossil fuels, biofuels represent a more environmentally friendly and sustainable fuel source. Plant or animal fats can be converted to biofuels through a process called transesterification. In particular, the storage molecule triacylglycerol (TAG), found in microscopic algae, is one of the most promising sources of fat for biofuel production, as microalgae are small, easy to grow, and reproduce quickly. Therefore, increasing the yield of TAG from microalgae could improve biofuel production processes. With this ultimate goal in mind, Professor Hiroyuki Ohta from the Tokyo Institute of Technology and colleagues investigated the conditions under which the model microalga Chlamydomonas reinhardtii produces more TAG. read more>
This research was published in The Plant Journal at 27 July 2019.
You can get the tools and databases that was the basis for metabolome analysis in this research from the following site. KOMICS (The Kazusa Metabolomics Portal)
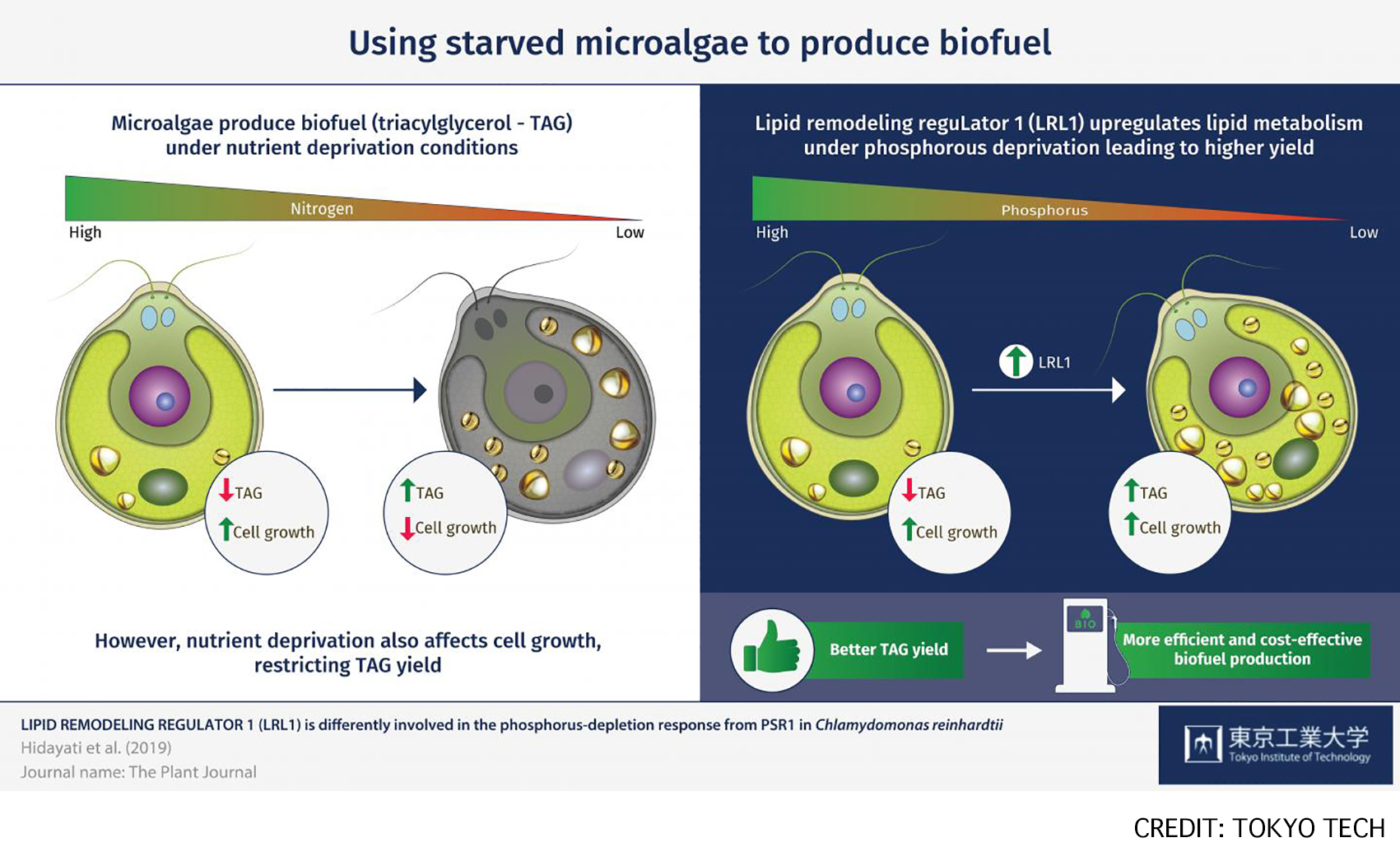
Fig: Microalgae are a promising source of biofuel feedstock, as they produce triacylglycerol (TAG) as a major storage lipid, especially under nutrient-depleted conditions. LRL1 was involved in the regulatory mechanism during the later stage of P-starvation in C. reinhardtii, as its regulation might depend on P-status, cell growth, and other factors.
Scaffolding system for three-dimensional organization of neurons in the developing neocortex
Press release
Memo1 Mediated Tiling of Radial Glial Cells Facilitates Cerebral Cortical Development
Naoki Nakagawa, Charlotte Plestant, Keiko Yabuno-Nakagawa, Jingjun Li, Janice Lee, Chu-Wei Huang, Amelia Lee, Oleh Krupa, Aditi Adhikari, Suriya Thompson, Tamille Rhynes, Victoria Arevalo, Jason L. Stein, Zoltán Molnár, Ali Badache, E. S. Anton
Neuron Published:July 02, 2019 DOI:10.1016/j.neuron.2019.05.049
Press release (In Japanese only)
In the mammalian neocortex, formation of distinct neuronal layers is important for precise information processing. During the embryonic brain development, neural progenitor cells not only generate neurons, but also play an important role in the neuronal layer formation. The process sprouted from the neural progenitor cell, termed radial process (Figure 1), is used as “guide” for migrating neurons. Each neural progenitor cell has a single radial process that extends toward the brain surface in a non-overlapping, regularly interspaced manner, therefore functioning as a “scaffolding system” for the neuronal migration and layer formation. However, the molecular and cellular mechanisms for the generation of this scaffolding system is not well understood.
A team of Dr. Naoki Nakagawa at the National Institute of Genetics and Dr. Eva S. Anton at the University of North Carolina School of Medicine identified mediator of cell motility 1 (Memo1) as a key gene for building this scaffolding system and revealed its role in the neuronal layer formation in the mouse neocortex.
By analyzing embryonic brains of Memo1-knockout mice, they found that radial processes of Memo1-deficient neural progenitor cells showed extensive branching and altered spatial distribution. These defects disrupted the scaffolding system of neural progenitor cells and resulted in neuronal misplacement and disorganized layers. These findings demonstrate that Memo1 is important for neural progenitor cells to form the regularly interspaced scaffolding system to organize cortical neurons into distinct neuronal layers (Figure 1).
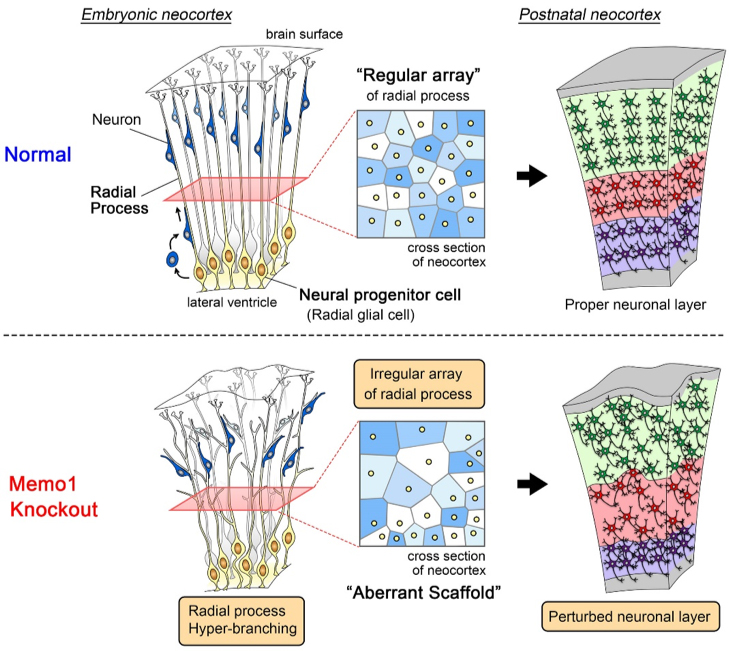
Figure 1: The scaffolding system of neural progenitor cells and neuronal layer formation
(Top) In the embryonic neocortex, neural progenitor cells are located at the ventricular surface and extend single radial processes toward the brain surface. Radial processes do not overlap with each other and are regularly interspaced. Newborn neurons migrate toward the superficial area by using this regular array of radial process as a “scaffold”, eventually forming the proper neuronal layer.
(Bottom) In the Memo1-knockout mouse neocortex, the absence of Memo1 function in neural progenitor cells causes hyper-branching and irregular distribution of radial processes. These phenotypes generate an aberrant scaffold, leading to perturbed neuronal layer formation.
Summer Holiday (Aug. 15,16)
NIG will be closed from August 15 and August 16, 2019 for summer holiday.
Thank you for your understanding and cooperation.
“Safe” cellular proliferation depending on “toxic” photosynthesis
Day/Night Separation of Oxygenic Energy Metabolism and Nuclear DNA Replication in the Unicellular Red Alga Cyanidioschyzon merolae.
Shin-ya Miyagishima, Atsuko Era, Tomohisa Hasunuma, Mami Matsuda, Shunsuke Hirooka, Nobuko Sumiya, Akihiko Kondo, Takayuki Fujiwara
mBio 10(4), e00833-19, 2019 DOI:10.1128/mBio.00833-19
Eukaryotes acquired chloroplasts through an endosymbiotic event in which a cyanobacterium or a unicellular eukaryotic alga was integrated into a previously nonphotosynthetic eukaryotic cell. Photosynthesis by chloroplasts enabled algae to expand their habitats and led to further evolution of land plants. However, photosynthesis causes greater oxidative stress than mitochondrion-based respiration. In seed plants, cell division is restricted to nonphotosynthetic meristematic tissues and populations of photosynthetic cells expand without cell division. Thus, seemingly, photosynthesis is spatially sequestrated from cell proliferation. In contrast, eukaryotic algae possess photosynthetic chloroplasts throughout their life cycle. Here we show that oxygenic energy conversion (daytime) and nuclear DNA replication (night time) are temporally sequestrated in C. merolae. This sequestration enables “safe” proliferation of cells and allows coexistence of chloroplasts and the eukaryotic host cell, as shown in yeast, where mitochondrial respiration and nuclear DNA replication are temporally sequestrated to reduce the mutation rate.
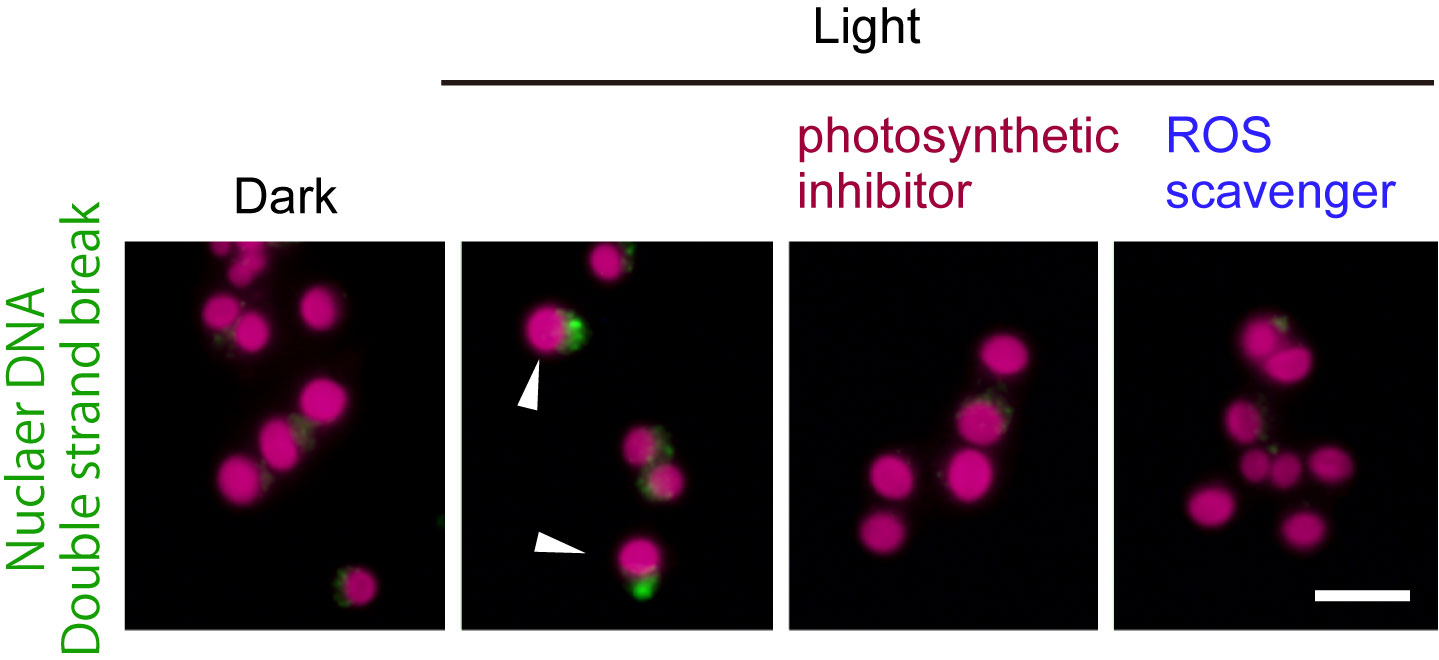
Figure1: The unicellular red alga Cyanidioschyzon merolae was cultured under a 12-h light / 12-h dark cycle. The cells early in the dark period (dark) were illuminated (light) either with or without DCMU (photosynthetic inhibitor) or TEMPOL (ROS scavenger). The green fluorescence indicates accumulation of MRE11 in the nucleus (DNA double strand break). The red is autofluorescence of the chloroplast. When the cells during the subjective night perform photosynthesis, the frequency of nuclear DSB (arrowheads) increases.
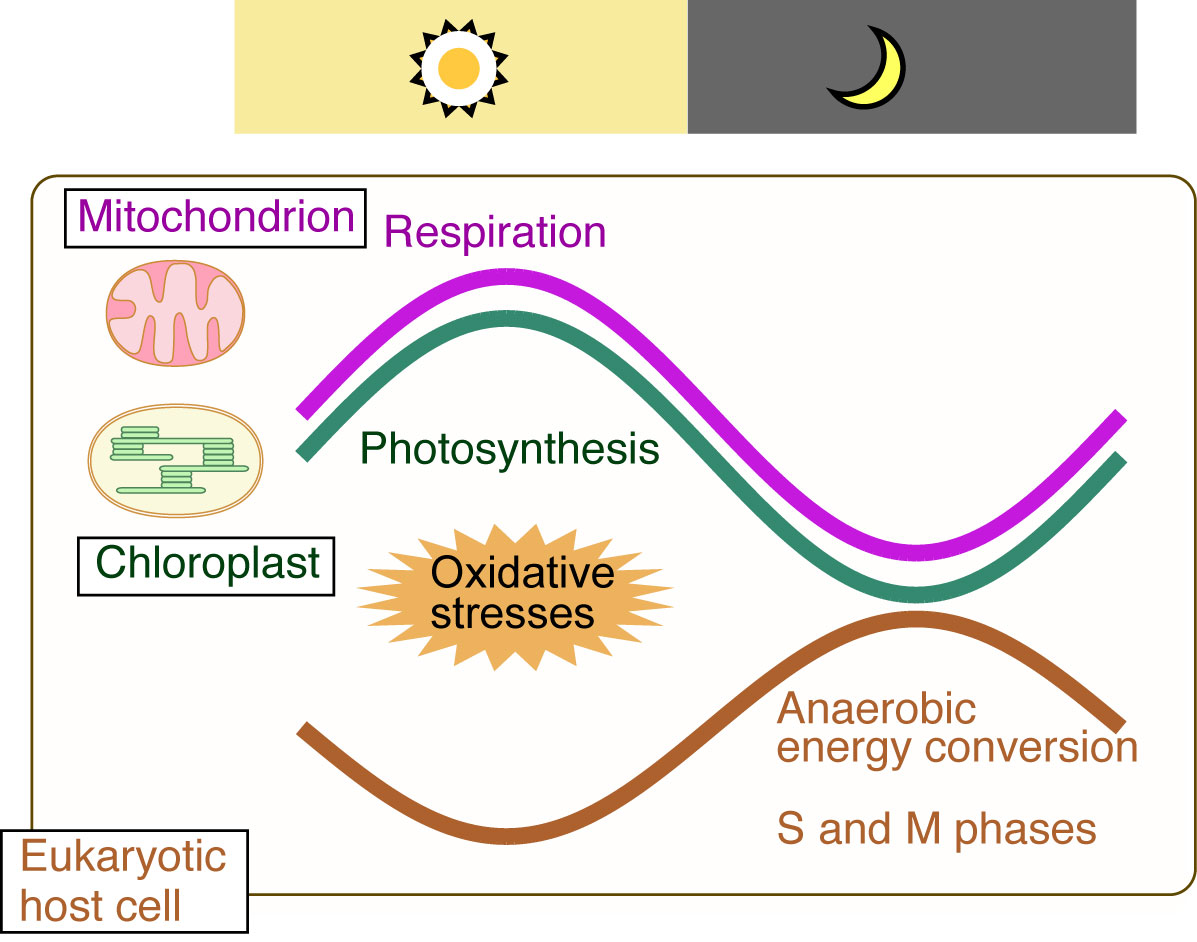
Figure2: The temporal separation of ROS-generating oxygenic energy metabolism by mitochondria and chloroplasts (endosymbiotic organelles) and nuclear DNA replication (eukaryotic host cell) ensures safe cell proliferation
Faculty member SHIMAMOTO at the Center for Frontier Research has been awarded tenure
NIG is proud to announce that an associate professor in the Center for Frontier Research has been awarded tenure as of July 1, 2019.
SHIMAMOTO, Yuta: Physics and Cell Biology Laboratory
Center for Frontier Research is an incubation center to simultaneously develop two elements: human resources and new research fields. Promising young scientists conduct research as principal investigator (tenure-track associate professor) to explore new frontiers in genetics and related areas, taking advantage of NIG’s research infrastructure and various support systems. Those who obtained tenure will establish new research divisions in NIG to lead the new fields that they contribute in creating.

- SHIMAMOTO, Yuta associate Professor
Polyploid genomes in bacteria: regulation of replication and the biological significance
Coordination of polyploid chromosome replication with cell size and growth in a cyanobacterium.
Ryudo Ohbayashi, Ai Nakamachi, Tetsuhiro Hatakeyama, Yu Kanesaki, Satoru Watanabe, Taku Chibazakura, Hirofumi Yoshikawa, and Shin-ya Miyagishima
mBio 10(2), e00510-19 DOI:10.1128/mBio.00510-19
Homologous chromosome number (ploidy) has diversified among bacteria, archaea, and eukaryotes over evolution. In bacteria, model organisms such as Escherichia coli possess a single chromosome. In contrast, other bacteria, including cyanobacteria, maintain multiple copies of individual chromosomes (polyploid). Although a correlation between ploidy level and cell size has been observed in bacteria and eukaryotes, it is poorly understood how replication of multi-copy chromosomes is regulated and how ploidy level is adjusted to cell size. Here we show that only one or a few multi-copy chromosomes are replicated at once in the cyanobacteria Synechococcus elongatus and that this restriction depends on regulation of DnaA activity. When cell growth rate was increased or decreased, DnaA level, DnaA activity, and the number of replicating chromosomes also increased or decreased in parallel, resulting in nearly constant chromosome copy number per unit cell volume. Thus, it is suggested that the stepwise replication of the genome enables cyanobacteria to maintain nearly constant gene copy number per unit cell volume.
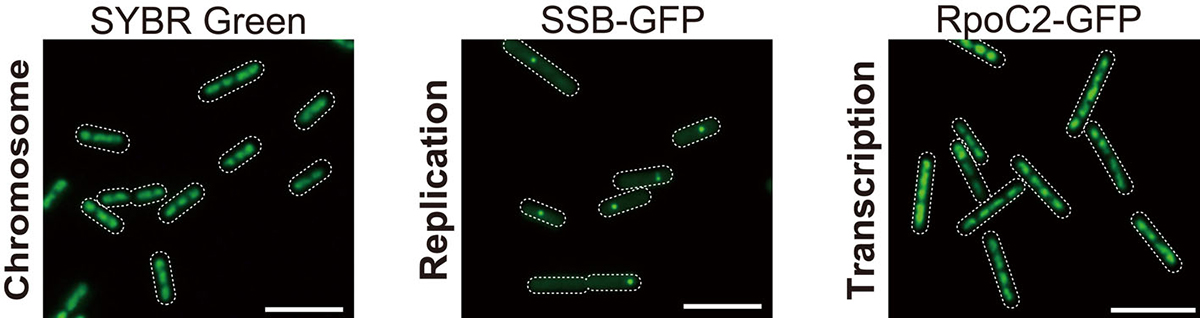
Figure. 1: Microscopic images of SYBR Green-stained nucleoids, SSB-GFP (replicating chromosomes), and RpoC2-GFP (transcribed chromosomes). All copies of chromosomes are transcribed while chromosomes are replicated one by one.
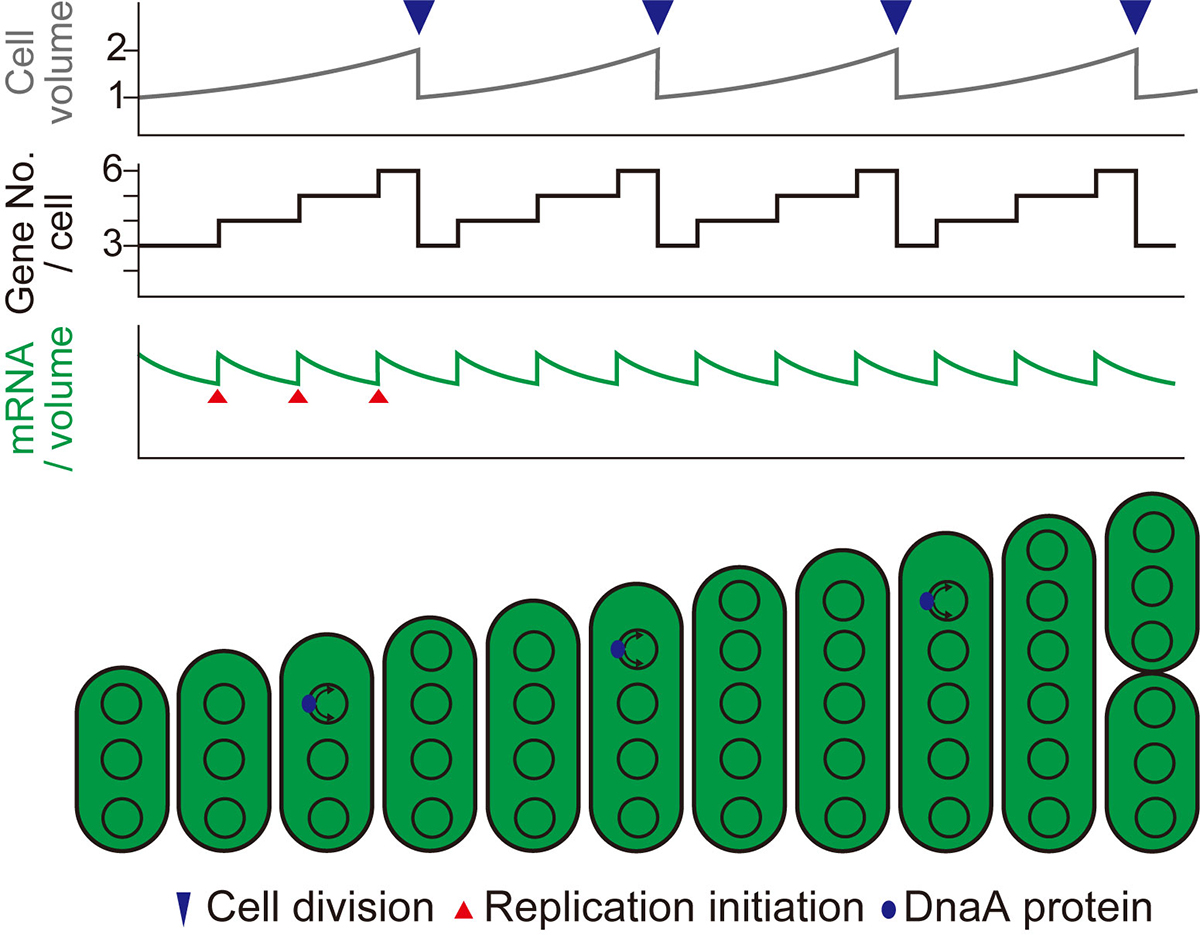
Figure. 2: Schematic diagrams showing changes in cell volume (upper), gene copy number per cell (middle), and mRNA level per unit volume (bottom) during cell growth in the case of stepwise (as observed in this study) replication of multi-copy chromosomes.
Identification of a key gene for freshwater colonization in fishes
Press release
A key metabolic gene for recurrent freshwater colonization and radiation in fishes.
Asano Ishikawa, Naoki Kabeya, Koki Ikeya, Ryo Kakioka, Jennifer N. Cech, Naoki Osada, Miguel C. Leal, Jun Inoue, Manabu Kume, Atsushi Toyoda, Ayumi Tezuka, Atsushi J. Nagano, Yo Y. Yamasaki, Yuto Suzuki, Tomoyuki Kokita, Hiroshi Takahashi, Kay Lucek, David Marques, Yusuke Takehana, Kiyoshi Naruse, Seiichi Mori, Oscar Monroig, Nemiah Ladd, Carsten J. Schubert, Blake Matthews, Catherine L. Peichel, Ole Seehausen, Goro Yoshizaki, and Jun Kitano.
Science 31 May 2019: Vol. 364, Issue 6443, pp. 886-889 DOI:10.1126/science.aau5656
EurekAlert! link about this artcle
Fishes are present in not only marine but also freshwater environments including rivers, streams, lakes, and ponds because ancestral marine fishes have colonized freshwater multiple times and diversified in the freshwater environment during evolution. However, only few fish lineages could colonize freshwater habitats. What enable some lineages to colonize freshwater?
Dr. Asano Ishikawa and Dr. Jun Kitano of Ecological Genetics Laboratory at the National Institute of Genetics, Japan, collaborated with researchers from Japan, Switzerland, USA, and Spain and asked this question using stickleback fishes (Fig. 1) as a model. They identified a key gene important for freshwater colonization, Fatty acid desaturase 2 (Fads2), encoding an enzyme involved in the synthesis of docosahexaenoic acid (DHA), a polyunsaturated fatty acid. DHA is a component of the cell membrane and is essential for growth and survival of fish. In aquatic ecosystems, marine-derived diets are rich in DHA, while diets in freshwater environments comprise limited DHA. They found that sticklebacks that successfully colonized freshwater have higher copy numbers of Fads2 and higher abilities to synthesize DHA than those that failed to colonize freshwater (Fig. 2). Analysis of publicly available whole-genome sequences of 48 fish species revealed higher Fads2 copy numbers in freshwater fish than in marine fish across diverse fish taxa.
This research was supported by JSPS KAKENHI (15H02418, 23113007, 16H06279, 26870824, 16K07469), JSPS PD (11J04816, 16J06812), Asahi Glass Foundation, Sumitomo Foundation, SNF grant (31003A 175614), and SNSF (31003A_163338, PDAMP3_123135).
This was published in Science at 2:00 p.m. U.S. Eastern Time on Thursday, 30 May 2019.

Fig: 1.A photo of a Japan Sea stickleback (Gasterosteus nipponicus) male. Photo by Yasuyuki Hata.
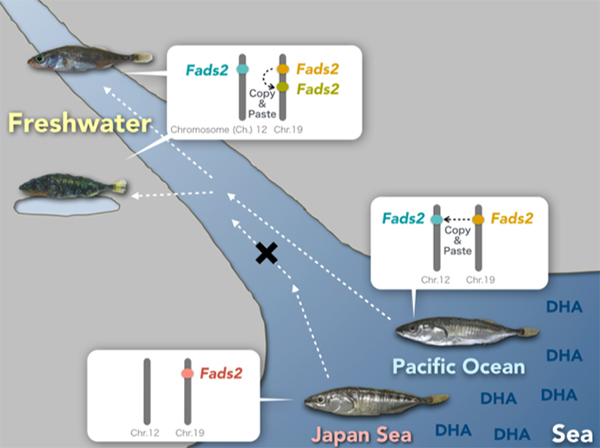
Fig: 2.Freshwater colonization and increase in Fads2 gene Japan Sea sticklebacks that could not colonize freshwater have only one Fads2 copy on Chromosome 19, while three-spined sticklebacks that could colonize have additional copy of Fads2 on Chromosome 12. Some freshwater populations of three-spined sticklebacks further duplicated Fads2 genes on Chromosome 19.
Robustness and flexibility of the chromosome segregation apparatus
Mechanically distinct microtubule arrays determine the length and force response of the meiotic spindle
Jun Takagi, Ryota Sakamoto, Gen Shiratsuchi, Yusuke T. Maeda, Yuta Shimamoto
Developmental Cell, Vol 49, pp 267-278, 2019 DOI:10.1016/j.devcel.2019.03.014
During cell division, our genome must be faithfully transmitted from the mother cell to daughter cells. This task is done by the spindle, the micron-sized bipolar force-generating machinery. Using a microfabricated force sensor and high-resolution microscopy, a team of Yuta Shimamoto at National Institute of Genetics and Yusuke Maeda at Kyushu University probed the local mechanical architecture of the spindle (Fig. 1A) and found its substantial heterogeneity; the equator and pole regions are stiff whereas its middle is more flexible and deformable (Fig. 1B). The spindle’s mechanical heterogeneity explains how this micron-sized structure can maintain its local functional architectures, such as the pole and the equator, required to pull chromosomes and determine the cell division axis, while adapting the overall structure to perturbations. Their physical approach will help understand the mechanisms ensuring the fidelity of chromosome segregation, which is essential for normal cell proliferation and embryonic development in humans.
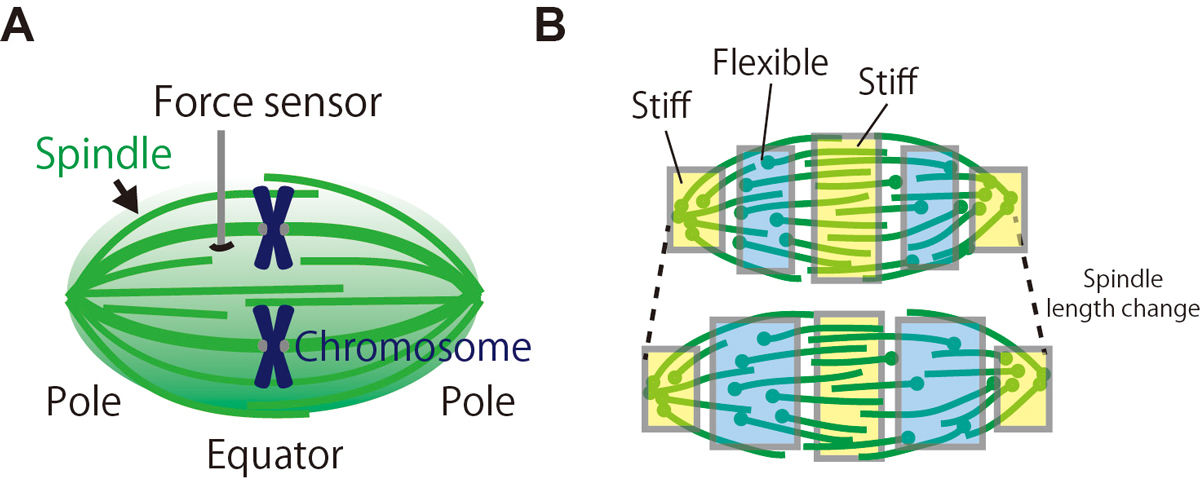
Figure: (A) The local mechanical architecture of the spindle was probed using a fiber-shaped microforce sensor. (B) The spindle was found to have a substantial mechanical heterogeneity (depicted in different colors), suggesting its robust yet adaptable nature required for error-free cell division.
Development of a CRISPR/Cas9-based tagging method and a degradation inhibitor for the auxin-inducible degron (AID) system
Generation of conditional auxin-inducible degron (AID) cells and tight control of degron-fused proteins using the degradation inhibitor auxinole
Aisha Yesbolatova, Toyoaki Natsume, Ken-ichiro Hayashi, Masato T. Kanemaki
Methods Available online 24 April 2019 DOI:10.1016/j.ymeth.2019.04.010
The auxin-inducible degron (AID) technology developed by the Kanemaki group is based on a plant-specific degradation pathway transplanted to non-plant cells. In human cells expressing a plant E3 ligase component, TIR1, a degron-fused endogenous protein is degraded within 15–45 min upon addition of the phytohormone auxin.
We demonstrated previously the generation of human HCT116 mutants in which the C terminus of endogenous proteins was fused with the degron by CRISPR–Cas9-based knock-in (Natsume et al. Cell Reports, 2016). In this study, A SOKENDAI student, Aisha Yesbolatova, developed new plasmids for N-terminal tagging and described a detailed protocol for the generation of AID mutants of human HCT116 and DLD1 cells (Figure 1).
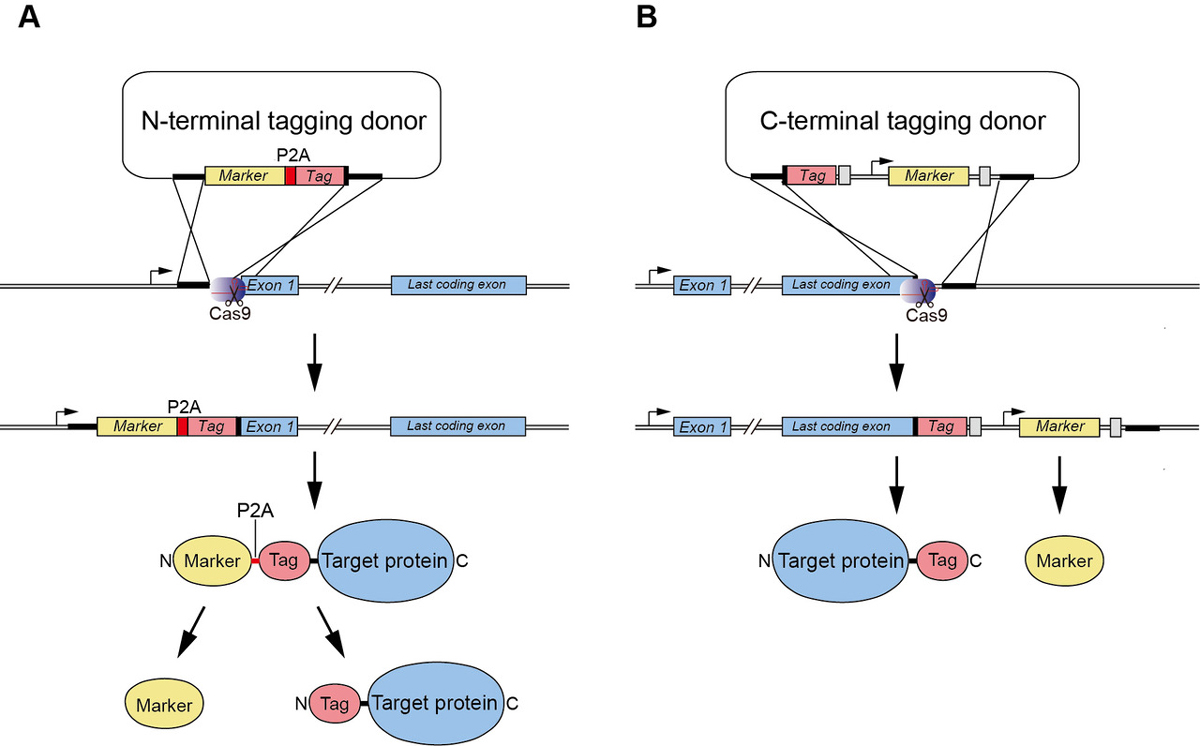
Figure1: (A) A new CRISPR/Cas9-based N-terminal tagging system. (B) C-terminal tagging.
Moreover, we report the use of a TIR1 inhibitor, auxinole, to suppress leaky degradation of degron-fused proteins (Figure 2). The addition of auxinole is also useful for rapid re-expression after depletion of degron-fused proteins.
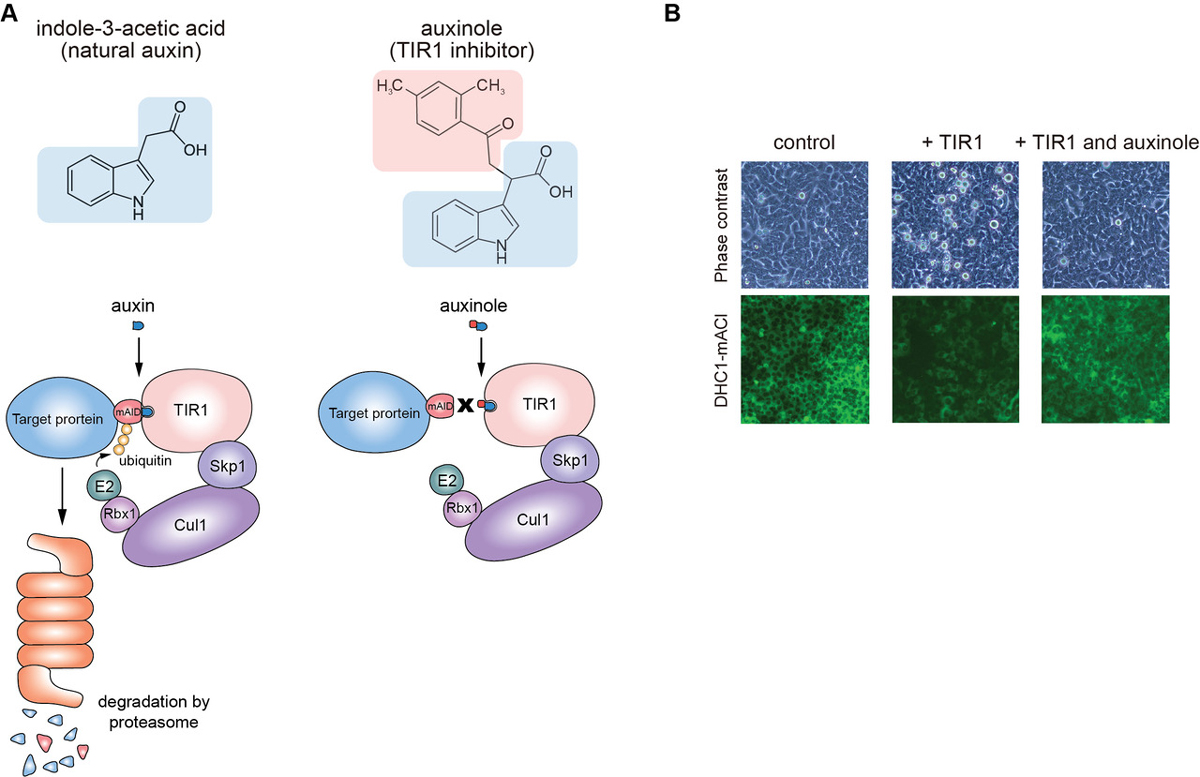
Figure2: (A) The structure of auxin and auxinole, and illustrations showing how these reagents work in the degradation pathway. (B) Suppression of leaky degradation in DHC1-tagged Tet-TIR1 cells. The cells were treated with only doxycycline to induce TIR1 expression or together with auxinole 48 h before microscopy.
We hope that these improvements enhance the utility of the AID technology for studying protein function in living human cells. All described plasmids are available from addgene and RIKEN-BRC.
This study was carried out as a collaboration with Prof. Ken-ichiro Hayashi of Okayama University of Science and was supported by JSPS KAKENHI (17K15068, 18H02170 and 18H04719), JST A-STEP (grant number AS2915150U), the Canon Foundation, the Asahi Glass Foundation, and the Takeda Science Foundation.
Evolutionary dynamics of developmental sequence in teleost lineage
Frequent nonrandom shifts in the temporal sequence of developmental landmark events during teleost evolutionary diversification
Fumihiro Ito, Tomotaka Matsumoto, Tatsumi Hirata
Evolution & Development 18 April 2019 DOI:10.1111/ede.12288
Development of multicellular organisms is characterized as a series of morphological events, whose temporal sequence, called as the developmental sequence, is well conserved in each individual species. In this study, we hypothesized that evolutionary changes in the sequence can be the basis for morphological diversifications, and examined the evolutionary dynamics of the developmental sequence in teleosts. Using the information from previous reports describing the development of 30 teleost species, we extracted the developmental sequences of 19 landmark events involving the formation of phylogenetically conserved body parts. Ancestral developmental sequences were then estimated by two different parsimony based methods. The phylogenetic comparisons of these sequences revealed that (1) the frequency of sequence changes differs among the events, (2) most of the sequence changes occurred as exchanges of temporally neighboring events, and (3) these changes in developmental sequences accumulate along the evolutionary time.
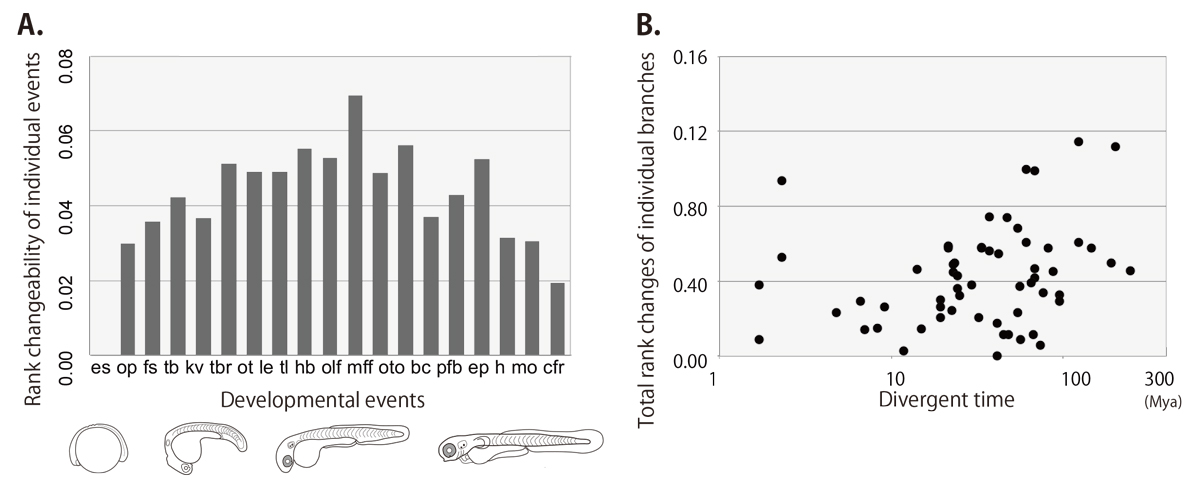
Figure: the rank changeability of individual developmental events (A) and the relationship between rank changes and divergent times (B) in teleost lineage estimated by the event-pairing method.
To divide into two? or three?, that is the question.
Choice between 1- and 2-furrow cytokinesis in Caenorhabditis elegans embryos with tripolar spindles
Tomo Kondo and Akatsuki Kimura
Molecular Biology of the Cell Published Online:20 Feb 2019 DOI:10.1091/mbc.E19-01-0075
Excessive centrosomes often lead to multipolar spindles, and thus probably to multipolar mitosis and aneuploidy. In Caenorhabditis elegans, approximately 70% of the paternal emb-27APC6 mutant embryonic cells contained more than two centrosomes and formed multipolar spindles. However, only 30% of the cells with tripolar spindles formed two cytokinetic furrows (Figure A, right). The rest formed one furrow, like normal cells (Figure A, left). To investigate the mechanism underlying this inconsistency, we conducted live-cell imaging in emb-27APC6 mutant cells. We observed that the chromatids were aligned only on two of the three sides of the tripolar spindle, and the angle of the tripolar spindle relative to the long axis of the cell correlated with the number of cytokinetic furrows (Figure B). Our numerical modeling showed that the combination of cell shape, cortical pulling forces, and heterogeneity of centrosome size determines whether cells with tripolar spindle form one or two cytokinetic furrows.
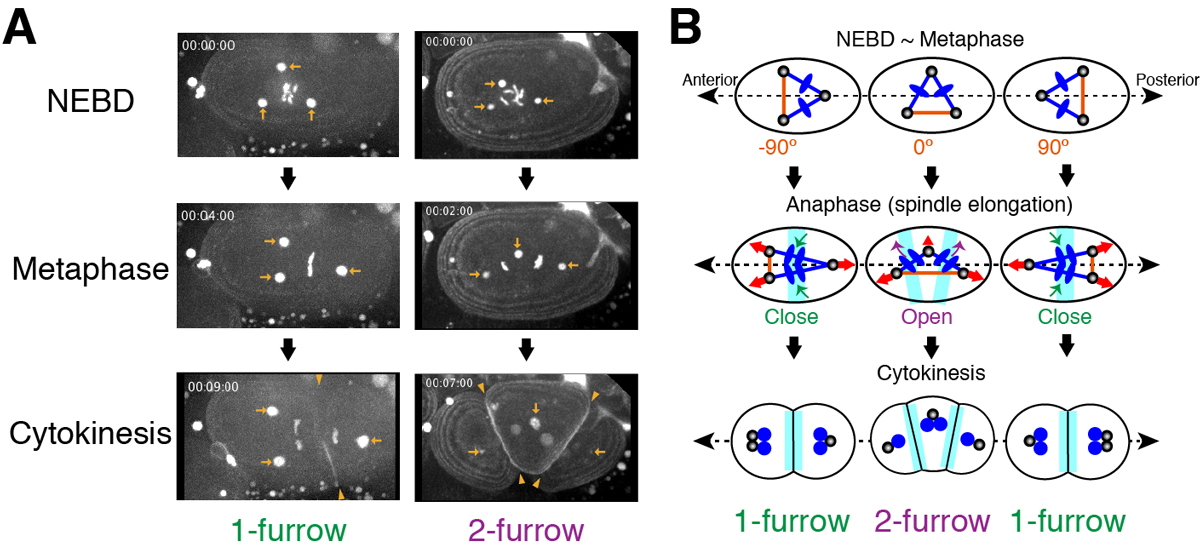
Figure:
(A) Images of a living C. elegans embryo with three centrosomes (arrows). In 1-furrow cytokinesis (left), a cleavage furrow (arrowheads) was observed between separated chromatids. In 2-furrow cytokinesis (right), two cleavage furrows (arrowheads) were observed.
(B) The proposed model showing how 1-furrow and 2-furrow cytokinesis are determined depending on the angle of tripolar spindle.
Transcriptional regulators of middle-wavelength sensitive visual photopigments
Six6 and Six7 coordinately regulate expression of middle-wavelength opsins in zebrafish
Yohey Ogawa, Tomoya Shiraki, Yoshimasa Asano, Akira Muto, Koichi Kawakami, Yutaka Suzuki, Daisuke Kojima and Yoshitaka Fukada
PNAS (2019) 116 (10) 4651-4660 DOI:10.1073/pnas.1812884116
Color discrimination in the vertebrate retina is mediated by a combination of cone cell types expressing UV- (SWS1), blue- (SWS2), green- (RH2), and red- (LWS) sensitive photoreceptive molecules (opsins). Although the tetrachromatic cone system is retained in most non-mammalian vertebrate lineages, the transcriptional mechanism underlying gene expression of cone opsins remains elusive.
In this study, we found that the retinal transcription factors, sine oculis homeobox 6 (Six6) and Six7, synergistically and positively regulate gene expression of zebrafish SWS2 and RH2 opsins. Larvae deficient for both of these transcription factors showed heavily impaired visually driven foraging behavior and were unable to compete for food when reared in a group with normal siblings. The results suggest that six6 and six7 play a pivotal role in blue- and green-light sensitivity and daylight vision.
This research was done by a team composed of Prof. Yoshitaka Fukada, Dr. Daisuke Kojima, and Dr. Yohey Ogawa at the University of Tokyo, and the Laboratory of Molecular and Developmental Biology at the National Institute of Genetics (Prof. Koichi Kawakami, Dr. Akira Muto, and Dr. Tomoya Shiraki).
This work was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) Grants (JP16J01681, JP16K20983, JP15K07144, JP18H04988, JP24227001, and JP17H06096).
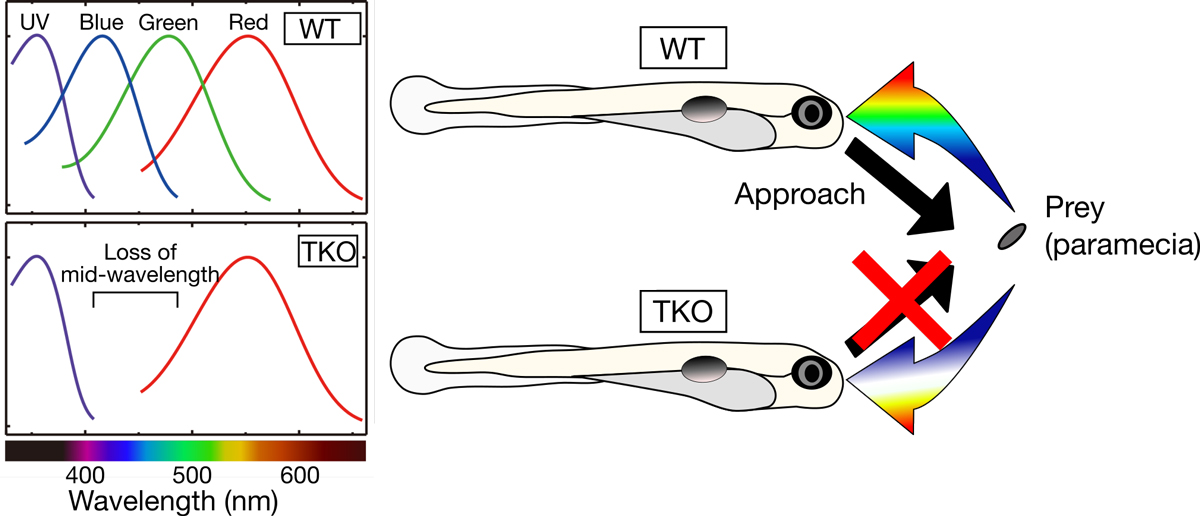
Figure: (Left) Zebrafish deficient for both of the Six7 and Six6 (TKO) lost gene expression of zebrafish SWS2 (blue) and RH2 (green) opsins, indicating that the TKO showed lower middle-wavelength sensitivity. (Right) The TKO showed heavily impaired visually driven foraging behavior and were unable to compete for food when reared in a group with normal siblings (WT).
OPEN HOUSE 2019
Time & Date
Saturday, April 6th, from 9:00am to 4:00pm (No Reservations Required, Free Admission)
Access
Free Shuttle Buses Available to the National Institute of Genetics from the North Exit of Mishima Station. (Service Time: 8:50am – 3:00pm)
Map
Map Free Shuttle Buses Available Parking around Mishima Station Available for Car Visitors.
No Pets Allowed except Service Dogs, No General Parking*
(*Disabled Parking Available on the Institute Premises)
Information
1111 Yata, Mishima, Shizuoka 411-8540, JAPAN TEL: +81-55-981-5873
Faculty member ODA at the Center for Frontier Research promoted to professor with tenure
NIG is proud to announce that an associate professor in the Center for Frontier Research has been awarded tenure and promoted to professor as of April 1, 2019.
ODA, Yoshihisa: Cell Dynamics and Signaling Laboratory
Center for Frontier Research is an incubation center to simultaneously develop two elements: human resources and new research fields. Promising young scientists conduct research as principal investigator (tenure-track associate professor) to explore new frontiers in genetics and related areas, taking advantage of NIG’s research infrastructure and various support systems. Those who obtained tenure will establish new research divisions in NIG to lead the new fields that they contribute in creating.

- ODA, Yoshihisa Professor
New assistant professor joins NIG
New assistant professor joins NIG as of April 1, 2019.
NEGISHI, Takefumi: Multicellular Organization Laboratory • Sawa Group
Mechanism that regulates an efficient switch from cell elongation to cell division.
Division-site localization of RodZ is required for efficient Z ring formation in Escherichia coli
Yusuke Yoshii, Hironori Niki, Daisuke Shiomi
Molecular Microbiology 2019 DOI:10.1111/mmi.14217
Cell elongation and cell division are controlled by different complexes. The mechanisms that promotes a switch from elongation to division have been still unclear. It has been recently reported that a direct interaction between MreB actin, which regulates elongation, and FtsZ tubulin, which regulates division, promotes the switching at the division site. We have analyzed RodZ that interacts with MreB. In this paper, we found that RodZ also localized on the division site depending on FtsZ, and that MreB localized to the division site depending on RodZ. When RodZ is not localized to the division site, the formation of the division ring (Z ring) composed of FtsZ was delayed. The localization of RodZ to the division site promotes the division ring formation and efficient cell division. This work was supported by KAKENHI and NIG-JOINT A.
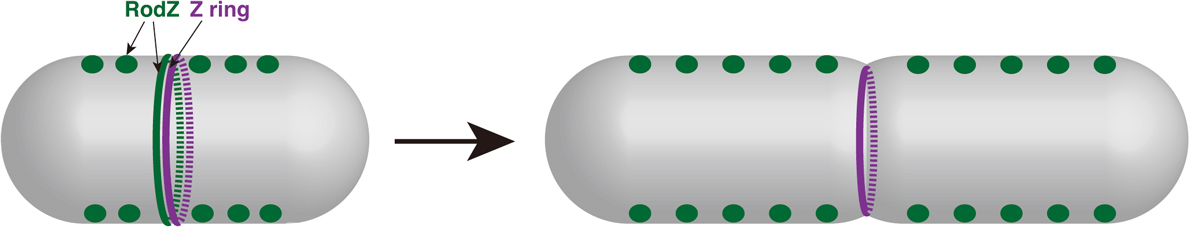
Figure: Division site localization of RodZ promotes an efficient formation of Z ring (i.e. switch from elongation to division).
Dynamic chromatin organization
Dynamic chromatin organization without the 30-nm fiber
Kazuhiro Maeshima, Satoru Ide and Michael Babokhov
Current Opinion in Cell Biology Volume 58, June 2019, Pages 95-104 DOI:10.1016/j.ceb.2019.02.003
Chromatin in eukaryotic cells is a negatively charged polymer composed of DNA, histones, and various associated proteins. Over the past ten years, our view of chromatin has shifted from a static regular structure to a dynamic and highly variable configuration. While the details are not fully understood yet, chromatin forms numerous compact domains that act as dynamic functional units of the genome in higher eukaryotes. By altering DNA accessibility, the dynamic nature of chromatin governs various genome functions including RNA transcription, DNA replication, and DNA repair/recombination. Based on new evidence coming from both genomics and imaging studies, we discuss the structural and dynamic aspects of chromatin and their biological relevance in the living cell.
This research was supported by JST CREST(JPMJCR15G2), JSPS Kakenhi (16H04746) and Takeda Science Foundation.
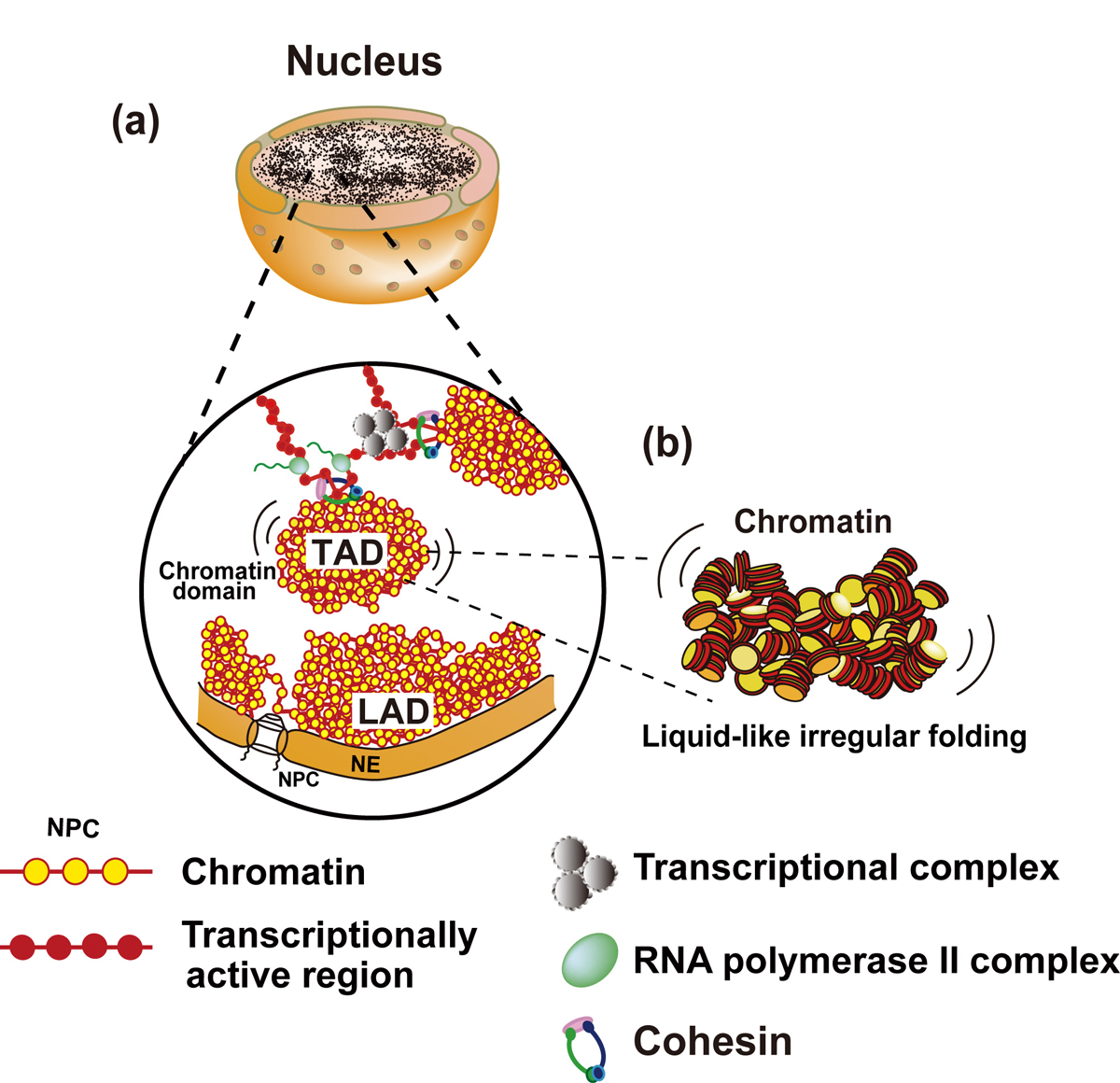
Figure: Interphase chromatin structure
(a and b) Chromatin consists of irregularly folded 10 nm fibers and forms numerous chromatin domains (e.g., topologically associating domains or contact/loop domains). The domains are formed by cohesin and nucleosome-nucleosome interactions. The compact domains could behave similar to a “liquid drop”. Binding of large transcriptional complexes (grey spheres) and RNA polymerase II (green spheres) might constrain movements of chromatin domains. Chromatin regions attached to nuclear envelop (NE) are called LADs (lamina-associated domains). NPC, nuclear pore complex; NE, nuclear envelop.
Transcription regulates dynamic movements of genomic DNA in living human cells
Press release
Single nucleosome imaging reveals loose genome chromatin networks via active RNA polymerase II
Ryosuke Nagashima, Kayo Hibino, S. S. Ashwin, Michael Babokhov, Shin Fujishiro, Ryosuke Imai, Tadasu Nozaki, Sachiko Tamura, Tomomi Tani, Hiroshi Kimura, Michael Shribak, Masato T.Kanemaki, Masaki Sasai, and Kazuhiro Maeshima
Journal of Cell Biology Published March 1, 2019 DOI:10.1083/jcb.201811090
Press release (In Japanese only)
The human body is composed of over forty trillion cells. Within each of these cells there is close to two meters of tightly packaged genomic DNA, the blueprint of life. Recently, there have been many advances in understanding how DNA is packaged and organized in the cell. In contrast, how DNA behaves mostly remains unknown.
In this recently published report, SOKENDAI graduate student Ryosuke Nagashima, assistant professor Kayo Hibino and professor Kazuhiro Maeshima of the National Institute of Genetics partnered with assistant professor S.S. Ashwin and professor Masaki Sasai of Nagoya University to study the movements of DNA in living cells using super resolution fluorescence microscopy (Figure 1A and B). Previously, it was commonly thought that the process of reading genomic information, known as transcription, would lead to more dynamic movements of DNA through the loosening of nucleosome packing. However, as Nagashima et al. report, transcription normally restricts DNA movement since the inhibition of transcription leads to increased DNA movements (Figure 1C). Furthermore, this work revealed that RNA polymerase II and other transcription-related factors form hubs to limit the motion of DNA (Figure 2). These results suggest that hubs may network the genome, restricting DNA movement to enable the efficient execution of transcription.
The results of this research have advanced our understanding as to how genetic information is acquired from DNA and may provide further clues concerning related disease states caused by abnormal changes to transcription.
This research is a result of a collaboration between the Genome Dynamics Laboratory of the National Institute of Genetics (Ryosuke Nagashima, Kayo Hibino, Michael Babokhov, Sachiko Tamura, Tadasu Nozaki, Ryosuke Imai, Kazuhiro Nagashima), the Nagoya University Department of Computational Science and Engineering (S.S. Ashwin, Shin Fujishiro, Masaki Sasai), the Cell Biology Center, Institute of Innovative Research, Tokyo Institute of Technology (Hiroshi Kimura), the Division of Molecular Cell Engineering of the National Institute of Genetics (Masato T. Kanemaki) and the Eugene Bell Center for Regenerative Biology and Tissue Engineering, Marine Biological Laboratory, Woods Hole (Michael Shribak, Tomomi Tani).
This research was supported by JST CREST(JPMJCR15G2), JSPS Kakenhi(16H04746), Takeda Science Foundation, RIKEN Pioneering Project, NIG-JOINT(2016-A2 (6)), SOKENDAI and National Institute of General Medical Sciences grant (R01-GM101701).
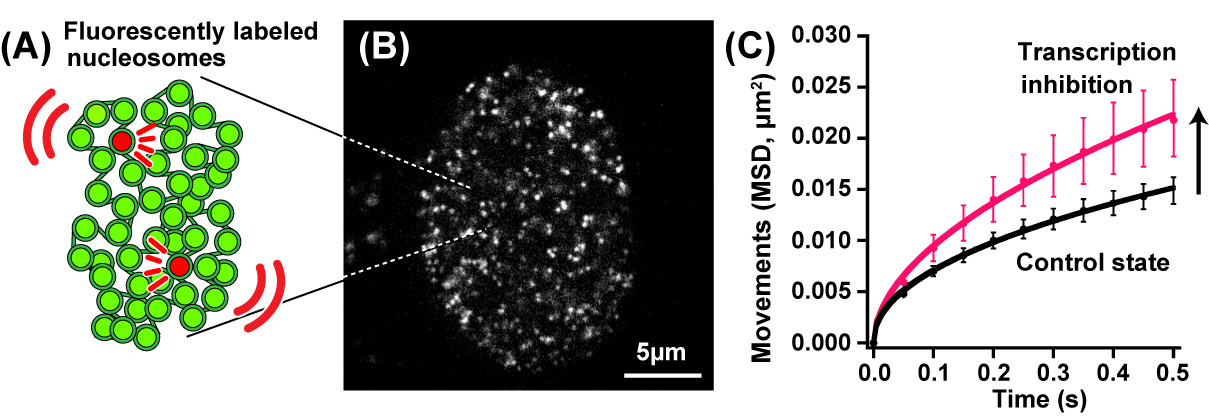
Fig.1. (A) A small fraction of nucleosomes was fluorescently labeled (red). (B) A single-nucleosomes image of a living human cell. (C) Genomic DNA motion, which is shown as mean square displacement, MSD), increased upon transcription inhibition.
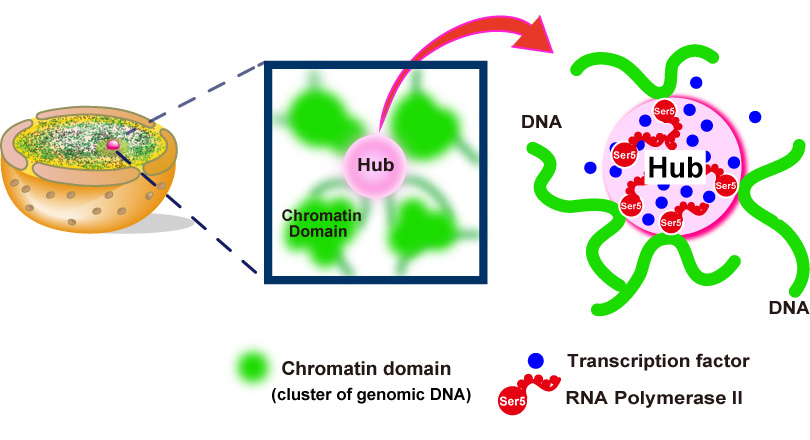
Fig.2. Hubs (pink spheres) of RNA polymerase II (red) and other components (blue spheres) of the gene transcription machinery constrain genomic DNA movements by connecting different genome regions together into an organized network.
Video1: Movie data (50 ms/frame) of single nucleosomes fluorescently labeled in a living human cell. Note that clear, well-separated dots and their movements were visualized.
Video2: Movie data (50 ms/frame) of single nucleosomes upon transcription inhibition. Note that greater movements of the dots are observed than those in Video1, suggesting the local genome dynamics increased upon transcription inhibition.
Mechanism of in vivo DNA cloning in Escherichia coli
Exonuclease III (XthA) Enforces In Vivo DNA Cloning of Escherichia coli To Create Cohesive Ends
Shingo Nozaki, Hironori Niki
Journal of Bacteriology 2018 Dec 10 PMID:30530516 DOI:10.1128/JB.00660-18
DNA fragments with overlapping homologous sequences at each end are joined as recombinant DNA molecules when introduced into Escherichia coli. However, it has long been unknown how the DNA fragments are joined in the cells. In this study, we demonstrated that single-stranded overhangs generated by 3’ to 5’ exonuclease activity of Exonuclease III (XthA) contribute to hybridization between the overlapping homologous sequences within the cells. Insights into the mechanism of in vivo DNA cloning, in combination with a simple transformation method, improve the ability of E. coli as a host for DNA manipulation.
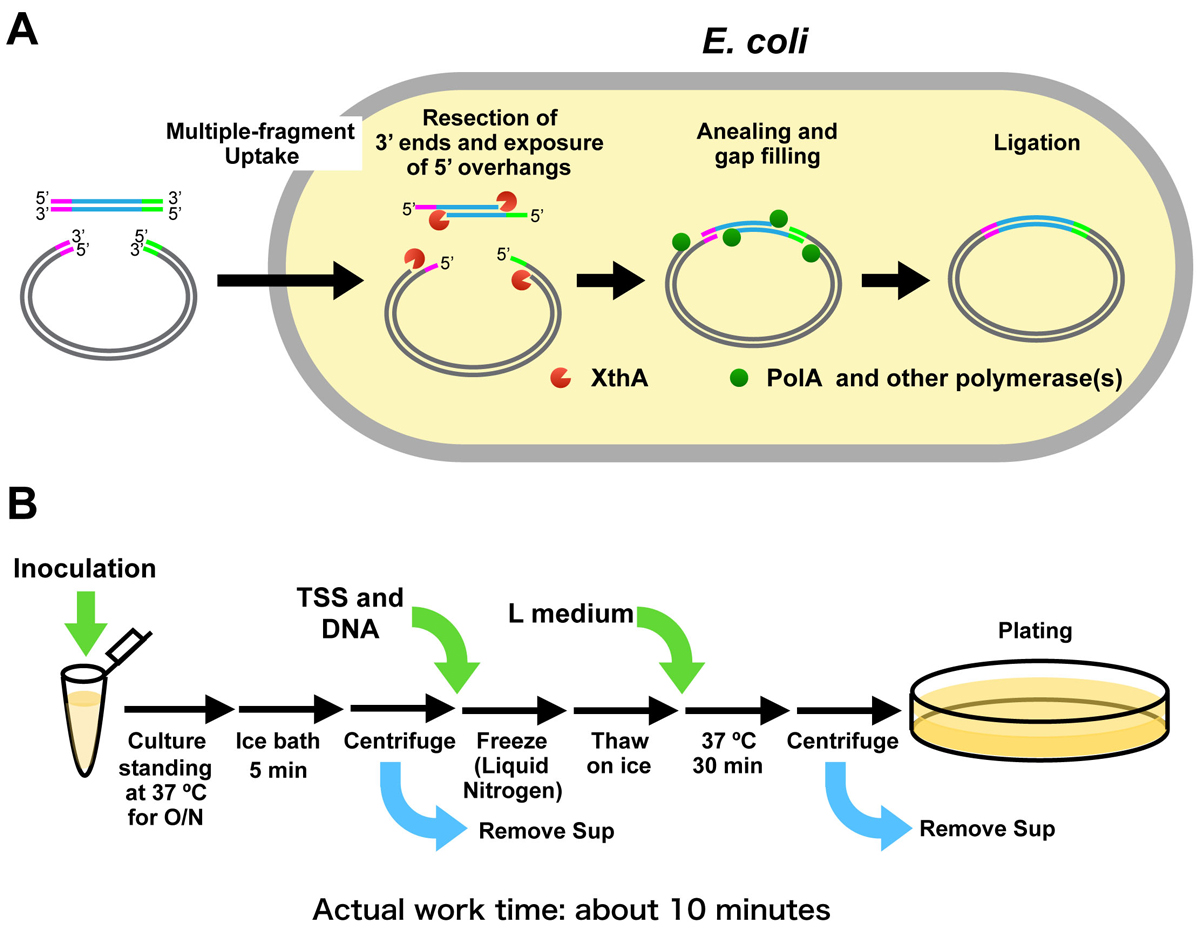
Figure:
(A) Model of the mechanism of iVEC (in vivo E. coli cloning)
(B) Work flow of “one-tube” transformation. Preparation of competent cells and introduction of DNA can be done with a single tube.
How sex pheromones diversify: lessons from yeast
Press release
Asymmetric diversification of mating pheromones in fission yeast
Taisuke Seike, Chikashi Shimoda, Hironori Niki
PLoS Biology published 22 Jan 2019 DOI:10.1371/journal.pbio.3000101
Press release (In Japanese only)
Many organisms including insects, amphibians and yeasts use sex pheromones for attracting individuals of the opposite sex, but what happens to sex pheromones as new species emerge? Our research group studies sex pheromones in the fission yeast Schizosaccharomyces pombe, revealing an “asymmetric” pheromone recognition system (Figure) in which one pheromone operates extremely stringently whereas the other pheromone is free to undergo a certain degree of diversification, perhaps leading to a first step towards speciation. Our findings contribute new insights into the evolutionary mechanisms underlying the diversification of pheromones. Organisms might have such systems for creating new versions of pheromones, allowing them to persist enough long in a population to evolve adaptations of receptors. Before a mutant is completely lost, a second suppressor mutation may occur to recover the first defect. Thus, the coevolution of pheromones/receptors can proceed step-by-step.
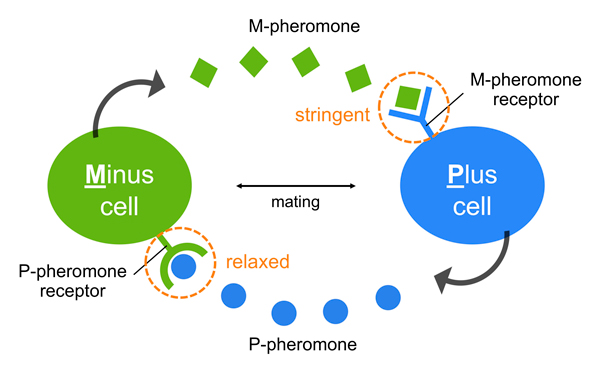
Figure: An asymmetric system of pheromone recognition in fission yeast
The specificity of M-pheromone is extremely stringent, whereas that of the P-pheromone is relatively relaxed; therefore, the diversity of the two sex pheromones might facilitate asymmetrically in nature, perhaps as a first step toward speciation in S. pombe.















