Shimamoto Group • Physics and Cell Biology Laboratory
Molecular and cellular biophysics dissecting chromosome dynamics regulation
Faculty
Research Summary
In most cells of our body, a variety of micron-sized structures, such as the nucleus and the mitotic spindle, assemble and function to control chromosome dynamics. Our laboratory uses advanced biophysical technologies, including intracellular micro/nanomanipulation, single-molecule imaging, and in vitro reconstitution, to visualize and manipulate such intracellular dynamics and unveil the intricated molecular mechanisms that ensure proper cell division and embryonic development.
Selected Publications
Sridhara A, Shimamoto Y. Microtubule choreography: spindle self-organization during cell division. Biophys Rev. 2024 Sep 30;16(5):613-624.
Saju A, Chen PP, Weng TH, Tsai SY, Tanaka A, Tseng YT, Chang CC, Wang CH, Shimamoto Y, Hsia KC. HURP binding to the vinca domain of β-tubulin accounts for cancer drug resistance. Nat Commun. 2024 Oct 14;15(1):8844.
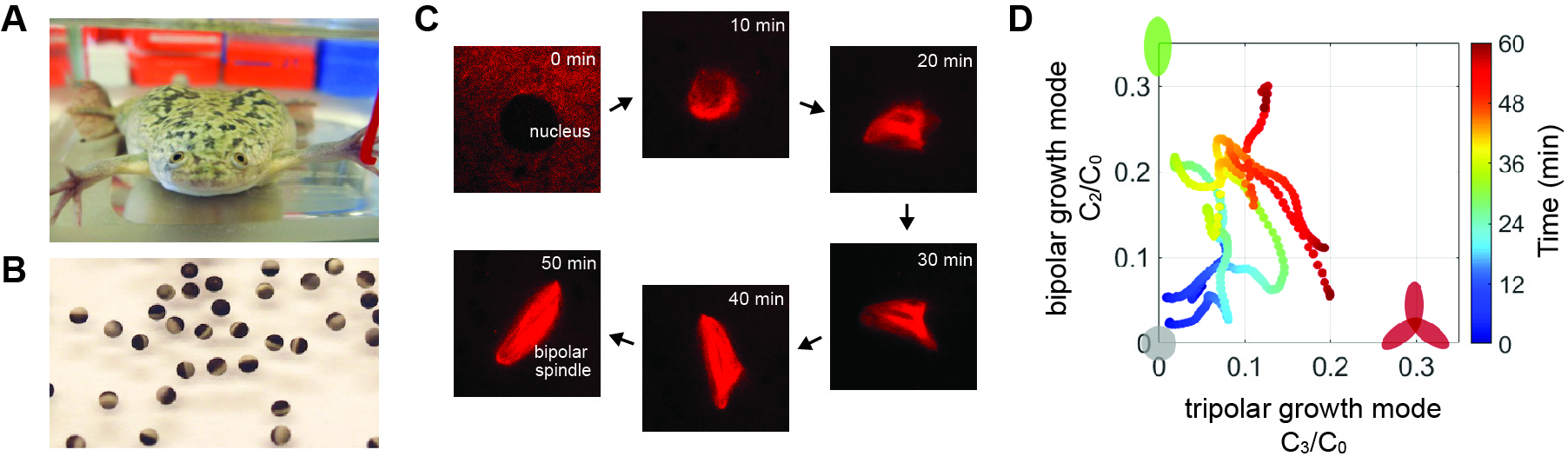
Fukuyama T, Yan L, Tanaka M, Yamaoka M, Saito K, Ti SC, Liao CC, Hsia KC, Maeda YT, Shimamoto Y. Morphological growth dynamics, mechanical stability, and active microtubule mechanics underlying spindle self-organization. Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2209053119.
Mori M, Yao T, Mishina T, Endoh H, Tanaka M, Yonezawa N, Shimamoto Y, Yonemura S, Yamagata K, Kitajima TS, Ikawa M. RanGTP and the actin cytoskeleton keep paternal and maternal chromosomes apart during fertilization. J Cell Biol. 2021 Oct 4;220(10):e202012001.
















