Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
A universal correlation between the density of the DNA and the length of mitotic chromosome
Cell Architecture Laboratory / Kimura Group
Scaling relationship between intra-nuclear DNA density and chromosomal condensation in metazoan and plant.
Hara Y, Adachi K, Kagohashi S, Yamagata K, Tanabe H, Kikuchi A, Okumura S-I, Kimura A.
Chromosome Science, 19, 43-49 (2016). DOI:10.11352/scr.19.43
Because the fundamental structure of chromosomes is conserved across eukaryotes, it might be assumed that an increase in the number of DNA base-pairs in a chromosome would lead to a corresponding increase in the physical length of chromosome. This does not appear to be the case, however. We compared the lengths of mitotic chromosome from several diverse species to determine the relationship between chromosome length, number of base-pairs, and the extent of chromosome packing. We found that all species share the same relationship among these, indicating that as base-pairs are added, chromosomes become more tightly packed so that the overall length increases less than expected. Our results suggest that instead of being related to the number of DNA base-pairs, chromosome length might be proportional to the surface area of the nucleus. This may be due to the need for the chromosomes to fit within a nuclear area known as the metaphase plate during mitosis, which occurs during cellular reproduction. This study provides insight into the features that drive the evolution of genome, chromosome, nucleus, and cell size and indicates that these characteristics are shared across eukaryotes.
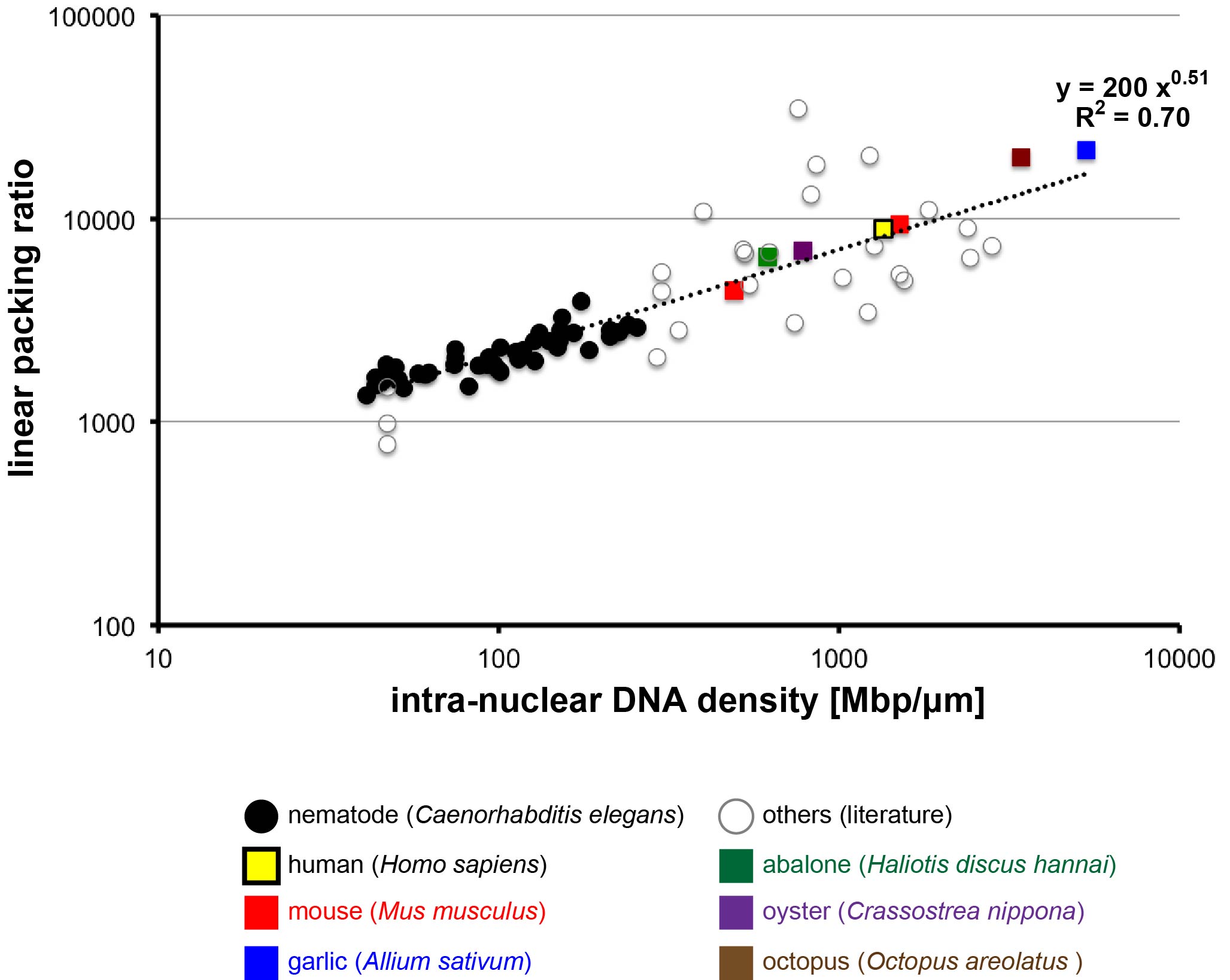
Figure. The degree of chromosome condensation (linear packing ratio) is correlated with intra-nuclear DNA density across species (Figure 1 of this paper). The double logarithmic plot of linear packing ratio against intra-nuclear DNA density is shown. The values obtained in this study are denoted with colored squares.
The timeline of chromosome organization in early embryogenesis
Cell Architecture Laboratory / Kimura Group
Reduction in chromosome mobility accompanies nuclear organization during early embryogenesis in Caenorhabditis elegans.
Arai R, Sugawara T, Sato Y, Minakuchi Y, Toyoda A, Nabeshima K, Kimura H, Kimura A.
Scientific Reports, 7, 3631 (2017). DOI:10.1038/s41598-017-03483-5
Many of us may imagine the DNA inside our cells as a jumble of noodles. However, DNA is more organized than this inside the nucleus, with chromosomes occupying distinct nuclear territories, for example. It is unclear though whether this organization is always present or whether it appears at some point during development after fertilization of the egg. We studied chromosome organization by observing the mobility of chromosomes inside in the nucleus in developing nematode embryos from the 2-cell to the 48-cell stage. We found that chromosome mobility decreases in 8-cell embryos, suggesting the initiation of chromosome organization at this point. Chromosome organization in the nucleus is important for gene expression and may have other purposes as well. For example, we found that in nematodes, the timing of chromosome organization coincides with the appearance of epigenetic marks, which regulate gene expression, and of a nuclear domain called the nucleolus. Now that we have identified the timeline of nuclear chromosome organization in nematodes, we will be able to conduct future studies to determine the factors responsible for initiating this organization.
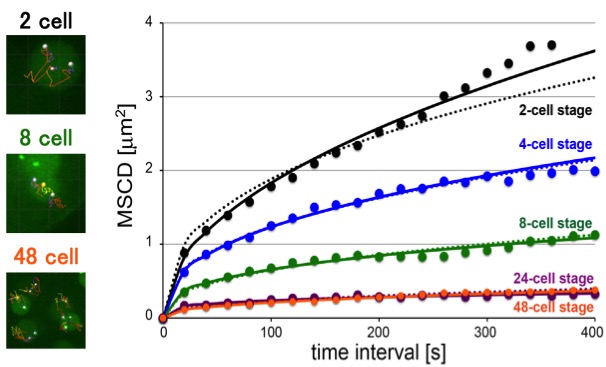
Figure. [Left images] Examples of the tracking (lines) of specific chromosomal loci (white dots) at indicated stages. The yellow dot reveals the center of the nucleus (not shown for the 48-cell stage). [Right graph] MSCD (mean squared change in distance) analyses of mobility: MSCD of the loci was plotted against the time interval (τ) for each stage. Some of the panels are identical as the panels in the paper (Arai et al., 2017)
Enhancer adoption changes limb morphology
![]()
Enhancer adoption caused by genomic insertion elicits interdigital Shh expression and syndactyly in mouse
Kousuke Mouri, Tomoko Sagai, Akiteru Maeno, Takanori Amano, Atsushi Toyoda, Toshihiko Shiroishi
PNAS Published online before print December 18, 2017 DOI:10.1073/pnas.1713339115
Pressrelease (In Japanese only)
Gene expression is regulated by tissue or organ-specific enhancer(s). Acquisition of new enhancers can alter the regulation of developmental genes, and may introduce morphological novelty in evolution. Reconfiguration between a gene and pre-existing enhancers of another gene by chromosomal rearrangement, which is referred to as “enhancer adoption”, is one possible source of new enhancers.
In this study, we re-examined an old mouse mutant named Hammer toe (Hm), which arose spontaneously over a half century ago and exhibits syndactyly with interdigital webbing. We revealed that a 150-kb non-coding genomic fragment that was originally located in chromosome 14 has been inserted into a genomic region upstream to Sonic hedgehog (Shh), located in chromosome 5. ATAC-seq and subsequent reporter assays in vivo revealed that the inserted fragment contains three interdigital enhancers to induce Shh expression in the interdigital regions in Hm. This ectopic Shh upregulates Chordin (Chrd), which in turn inhibits BMP signaling, and eventually results in syndactyly and web formation. Since the donor fragment residing in chromosome 14 has enhancer activity to induce interdigital gene expression, the Hm mutation appears to be an archetypal case of enhancer adoption. Our series of genomic deletion induced by the CRISPR-Cas9 system revealed that three enhancers in 150-kb fragment induce syndactyly in a cooperative manner. Acquisition of the combination of enhancers may have important role for the enhancer adoption.
This study was carried out as a collaboration of Kousuke Mouri, Tomoko Sagai, Akiteru Maeno, Takanori Amano and Toshihiko Shiroishi of Mammalian Genetics Laboratory, and Atsushi Toyoda of Comparative Genomics Laboratory, NIG. This study was supported by JSPS KAKENHI JP15J06985, JP17K15162 and JP17K19411.
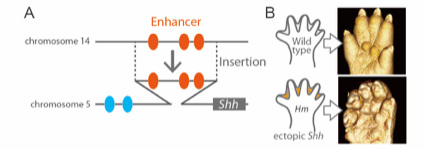
Fig. 1 (A) Hm has an insertion into the upstream region of Shh gene. Inserted fragment includes three interdigital enhancers. (B) X-ray micro-CT images of limb (P0). Shh is ectopically expressed in the interdigital region, and causes interdigital webbing in Hm.
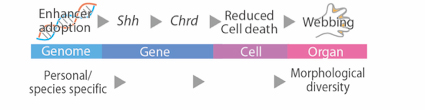
Fig. 2 The process that the morphological alteration in Hm was caused by the enhancer adoption.
You can see the several data of micro CT-scan which is one of the methods for this research below.
- NIG 3d Imaging Room(Facebook)
- 3D Imaging Room in NIG(sketchfab)
You can read the short review for this article.
You can read our news release in EurekAlert! about this article.
Protocadherin-αC2 is required for diffuse projections of serotonergic axons
Division of Neurogenetics / Iwasato Group
Protocadherin-αC2 is required for diffuse projections of serotonergic axons
Shota Katori, Yukiko Noguchi-Katori, Atsushi Okayama, Yoshimi Kawamura, Wenshu Luo, Kenji Sakimura, Takahiro Hirabayashi, Takuji Iwasato & Takeshi Yagi
Scientific Reports, 7, Article number: 15908 (2017) DOI:10.1038/s41598-017-16120-y
Serotonergic axons extend diffuse projections throughout various brain areas, and serotonergic system disruption causes neuropsychiatric diseases. Loss of the cytoplasmic region of protocadherin-α (Pcdh-α) family proteins, products of the diverse clustered Pcdh genes, causes unbalanced distributions (densification and sparsification) of serotonergic axons in various target regions. However, which Pcdh-α member(s) are responsible for the phenotype is unknown. Here we demonstrated that Pcdh-αC2 (αC2), a Pcdh-α isoform, was highly expressed in serotonergic neurons, and was required for normal diffusion in single-axon-level analyses of serotonergic axons. The loss of αC2 from serotonergic neurons, but not from their target brain regions, led to unbalanced distributions of serotonergic axons. Our results suggest that αC2 expressed in serotonergic neurons is required for serotonergic axon diffusion in various brain areas. The αC2 extracellular domain displays homophilic binding activity, suggesting that its homophilic interaction between serotonergic axons regulates axonal density via αC2’s cytoplasmic domain.
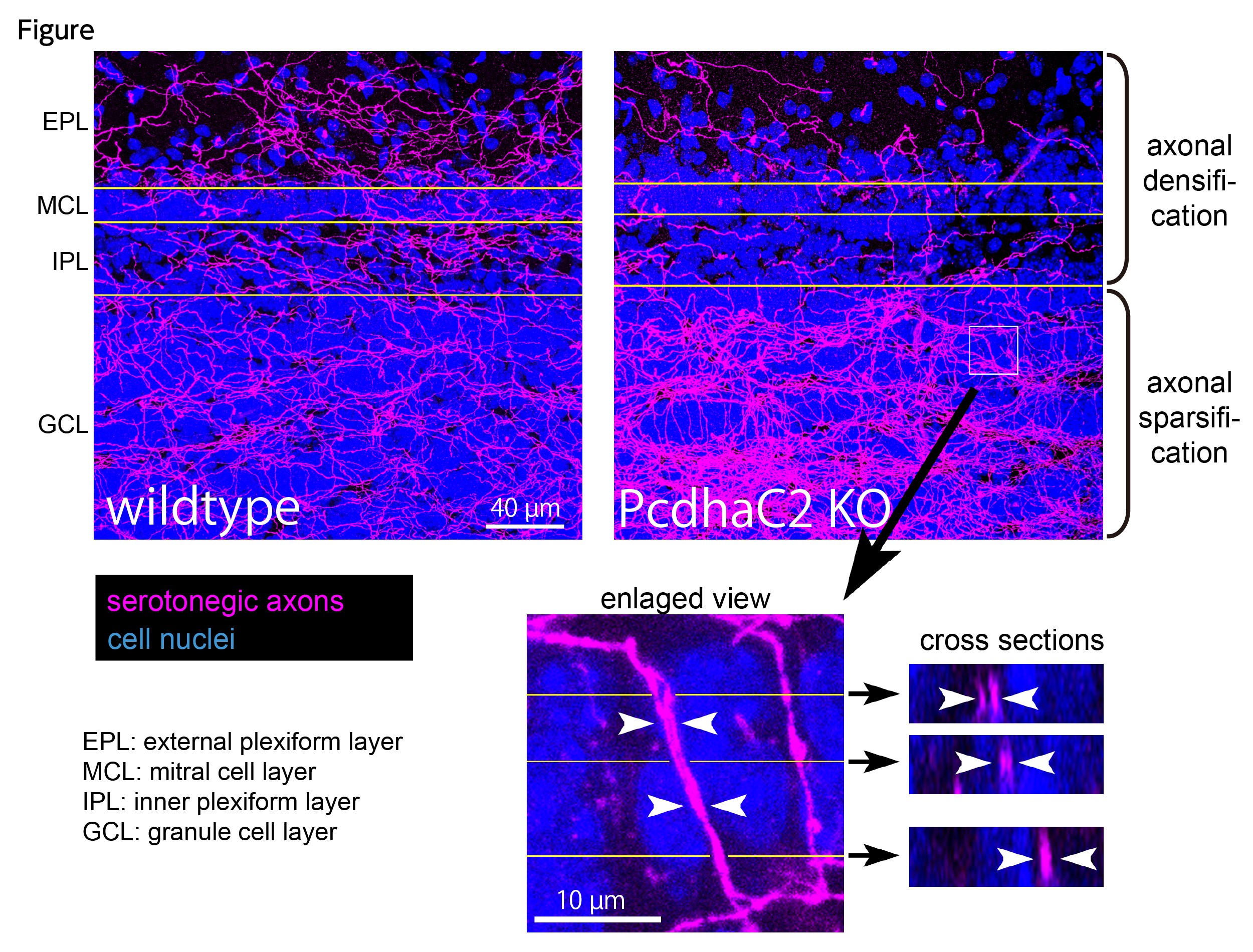
Figure. Serotonergic axons in the olfactory bulb. Wildtype mice (left) show almost uniform distributions in serotonergic axons (magenta) in all layers. In contrast, protocadherin-α C2 deficient mice (right) exhibit unbalanced serotonergic-axon distributions (densification in granule cell layer and sparsification in the other layers). In the granule cell layer (box), fasciculating serotonergic axons are observed.
Source: Scientific Reports 7 Article number: 15908(2017)
DOI: 10.1038/s41598-017-16120-y
Revisiting the ‘Chemotropic Theory’ of neural wiring
Division of Brain Function / Hirata Group
Netrin-1 Derived from the Ventricular Zone, but not the Floor Plate, Directs Hindbrain Commissural Axons to the Ventral Midline
Kenta Yamauchi, Maya Yamazaki, Manabu Abe, Kenji Sakimura, Heiko Lickert, Takahiko Kawasaki, Fujio Murakami, and Tatsumi Hirata
Scientific Reports, DOI:10.1038/s41598-017-12269-8
In this study, we have provided evidence that Netrin-1 (Ntn1) from the ventricular zone (VZ), but not the floor plate (FP), a glial structure occupying the ventral midline (VM), guides hindbrain commissural axon (CA) to the VM. Our results are incompatible with the prevailing view that Ntn1 is a chemoattractant for CAs, rather suggest a novel mechanism that VZ-derived Ntn1 directs CAs to the VM by its local actions.
More than a century ago, based on his observations of CA growth towards the VM, Ramón y Cajal proposed the ‘chemotropic theory’, attraction of growth cones by a long-range diffusible cue emanating from their targets. Ntn1 emanating from the VM has been assumed as the chemoattractant. Consistent with this view, Ntn1 is expressed in the VM and can attract CAs at a distance in vitro. However, Ntn1 is expressed in the vicinity of the CA path, the VZ, in addition to the FP, raising an alternative possibility that Ntn1 of extra-FP origin directs CAs to the VM. To test this possibility, we generated Ntn1 FP conditional mutant (Ntn1FP-Ko) and VZ conditional mutant mice (Ntn1VZ-Ko). If Ntn1 acts CAs as a long-range diffusible chemoattractant, CA guidance to the VM should be disrupted in Ntn1FP-Ko mice. Contrary to the prediction, deletion of Ntn1 from the VZ highly disrupted CA guidance to the VM, whereas deletion of Ntn1 from the FP had little impact on it (Figure). Our results fail to support the chemotropic theory proposed by Ramón y Cajal, and rather suggest that local actions of Ntn1 from the VZ direct CAs to the VM.
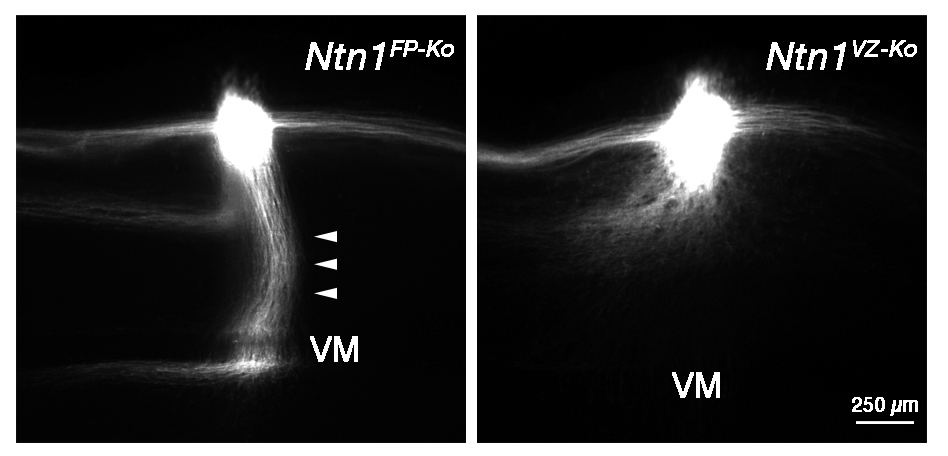
Hindbrain CA growth in Ntn1FP-Ko and Ntn1VZ-Ko mice. Deletion of Ntn1 from the VZ highly disrupts CA guidance to the VM (right), whereas deletion of Ntn1 from the FP has little impact on it (left, arrowheads). CA is labeled with a fluorescent lipophlic dye, DiI.
A new laboratory established in the Center for Frontier Research
Fumi KUBO joined the Center for Frontier Science as of December 1, 2017.
KUBO, Fumi:Center for Frontier Research,Systems Neuroscience Laboratory

- KUBO, Fumi
Associate Professor
Center for Frontier Research is an incubation center to simultaneously develop two elements: human resources and new research fields. Promising young scientists conduct research as principal investigator (tenure-track associate professor) to explore new frontiers in genetics and related areas, taking advantage of NIG’s research infrastructure and various support systems.
Development of organs in living whole embryo/larval grafts in zebrafish
Model Fish Genomics Resource Laboratory / Sakai Group
Development and growth of organs in living whole embryo and larval grafts in zebrafish
Toshihiro Kawasaki, Akiteru Maeno, Toshihiko Shiroishi & Noriyoshi Sakai
Scientific REPORTS, 7: 16508 DOI:10.1038/s41598-017-16642-5
Age-related systemic environments influence neurogenesis and organ regeneration of heterochronic parabiotic partners; however, the difficulty of manipulating small embryos prevents the effects of aged systemic environments on primitive organs at the developmental stage from being analysed.
Here, we describe a novel transplantation system to support whole living embryos/larvae as grafts in immunodeficient zebrafish by the intrusion of host blood vessels into the grafts, allowing bodies similar to those of heterochronic parabiosis to be generated by subcutaneous grafting. Although grafted embryos/larvae formed most organs, the liver, testes and heart developed insufficiently or even occasionally disappeared. Removal of host germ cells stimulated testis development in grafted embryos. These results indicate that primitive testes are susceptible to the systemic environments that originated from the germ cells of aged hosts and imply that the primitive liver and heart are similar. This unique transplantation system will lead to new insights into the age-related systemic environments that are crucial for organogenesis in vertebrates.
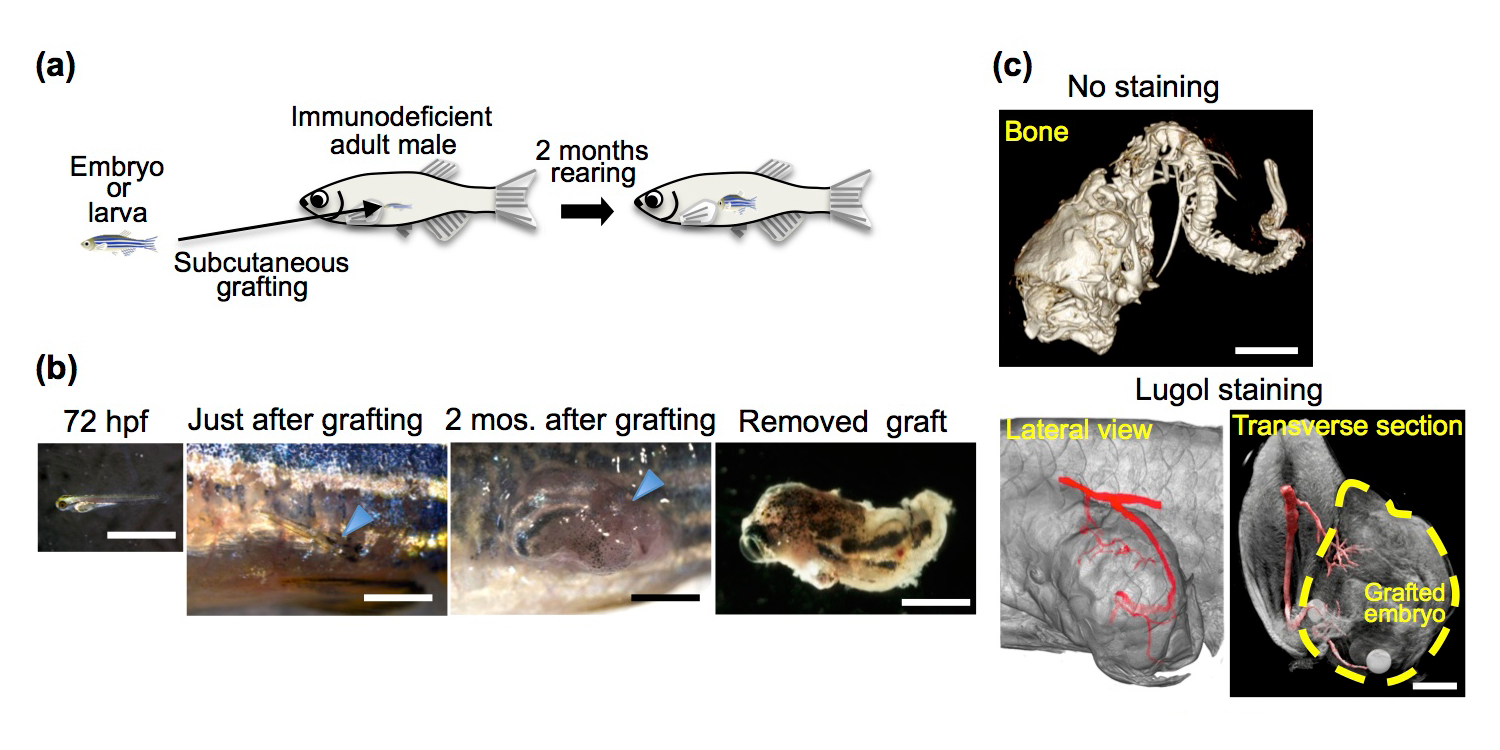
Development of grafted 72 hpf embryos in rag1 mutant hosts. (a) Schematic of whole-embryo transplantation. (b) Representative 72 hpf embryos and grafted embryos. (c) µ-CT imaging of the grafted embryo 2 months after transplantation. Cranial bones, vertebrae (No staining) and a vascular structure (Lugol staining) were observed in the grafted embryos.
You can see the several data of micro CT-scan which is one of the methods for this research below. NIG 3d Imaging Room (facebook)
3D Imaging Room in NIG(sketchfab)
An asymmetric attraction model for the diversity and robustness of cell arrangement in nematodes
Press release
An asymmetric attraction model for the diversity and robustness of cell arrangement in nematodes
Kazunori Yamamoto, Akatsuki Kimura
Development 2017 144: 4437-4449; DOI:10.1242/dev.154609
Pressrelease (In Japanese only)
During early embryogenesis in animals, cells are arranged into a species-specific pattern in a robust manner. Diverse cell arrangement patterns are observed, even among close relatives (Fig. A). In the present study, we evaluated the mechanisms by which the diversity and robustness of cell arrangements are achieved in developing embryos. We successfully reproduced various patterns of cell arrangements observed in various nematode species in Caenorhabditis elegans embryos by altering the eggshell shapes (Fig. B). The findings suggest that the observed diversity of cell arrangements can be explained by differences in the eggshell shape. Additionally, we found that the cell arrangement was robust against eggshell deformation. Computational modeling revealed that, in addition to repulsive forces, attractive forces are sufficient to achieve such robustness (Fig. C). The present model is also capable of simulating the effect of changing cell division orientation. Genetic perturbation experiments demonstrated that attractive forces derived from cell adhesion are necessary for the robustness. The proposed model accounts for both diversity and robustness of cell arrangements, and contributes to our understanding of how the diversity and robustness of cell arrangements are achieved in developing embryos.
This research article was published in the special issue of Development ‘On Growth and Form – 100 years on’, inspired by the seminal work of D’Arcy Thomson. See the authors’ comments:
Kazunori Yamamoto, the first author of the paper received Morishima Award for this research.
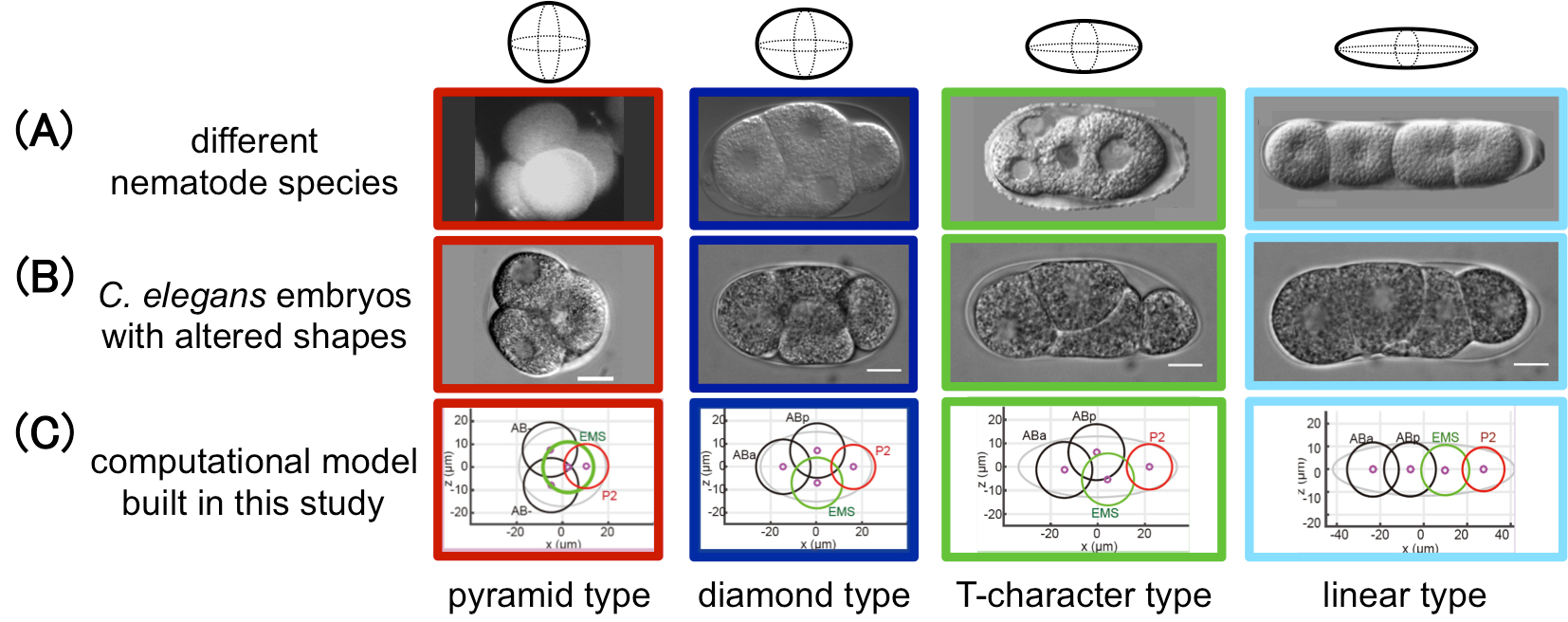
Figure: This study successfully reproduced the cell arrangement patterns of various nematode species (A) using C. elegans by altering the shape of the eggshell (B). A computational model was also built in this study that reproduces the diversity and robustness of the cell arrangement patters (C). In (A), the patterns of Enoplus brevis (red, image from Schulze and Shierenberg, Evodevo 2, 18, 2011), C. elegans (blue, this study), Cephalobus sp. (green, Goldstein, Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 1521-1531, 2001), and Belonolaimus sp. (light blue, Goldstein, 2001) are shown.
Source: Development 2017 144: 4437-4449
DOI: 10.1242/dev.154609
Conditional Degrons for Controlling Protein Expression at the Protein Level
Division of Molecular Cell Engineering / Kanemaki Group
Conditional Degrons for Controlling Protein Expression at the Protein Level
Toyoaki Natsume and Masato T. Kanemaki
Annual Review of Genetics, DOI:https://doi.org/10.1146/annurev-genet-120116-024656
It is a common genetic analysis in many organisms to see the phenotype after depletion of a protein of interest. Technologies such as siRNA and conditional gene knockout have been employed for this purpose. However, these do not deplete a protein of interest directly. It is, therefore, the depletion effect is indirect and it takes for a relatively long time to deplete a protein of interest, depending on the half-life of the protein. This sometime causes a problem because the phenotype can be complicated by a secondary effect that was initiated by a primary defect.
Recently, conditional degron technologies, by which a degron-fused protein can be induced for rapid degradation, are seizing the attention. Protein depletion by the degron technologies work directly at the protein level, so that it is possible to analyze the direct effect after rapid protein depletion. We developed the auxin-inducible degron technology by which a degron-fused protein can be controlled by a plant hormone auxin. Others used temperature, small chemicals, light, or the expression of another protein for the similar purpose. We wrote a review paper explaining the development history of conditional degrons and their characteristics.
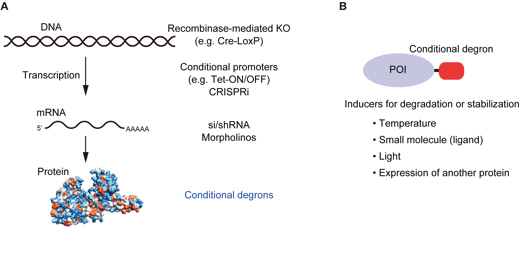
(A) Conditional technologies for controlling protein expression. From the level of gene to protein, various technologies have been developed. Conditional degrons work at the protein level. (B) Conditional degrons can be controlled by temperature, small chemicals, light, or the expression of another protein.
A novel mechanism that regulates decondensation of mitotic chromosomes
Microbial Genetics Laboratory / Niki Group
Release of condensin from mitotic chromosomes requires the Ran-GTP gradient in the reorganized nucleus.
Keita Aoki and Hironori Niki
Biology Open, November 15; 6(11):1614-1628, 2017 DOI:10.1242/bio.027193.
After mitosis, nuclear reorganization occurs together with decondensation of mitotic chromosomes and reformation of the nuclear envelope, thereby restoring the Ran-GTP gradient between nucleus and cytoplasm. The Ran-GTP gradient is dependent on Pim1/RCC1. Interestingly, a defect in Pim1/RCC1 in Schizosaccharomyces pombe causes post-mitotic condensation of chromatin, namely hyper-condensation, suggesting a relationship between the Ran-GTP gradient and chromosome decondensation. However, how Ran-GTP interacts with chromosome decondensation is unresolved. To examine this interaction, we used Schizosaccharomyces japonicus, which is known to undergo partial breakdown of the nuclear membrane during mitosis. We found that Pim1/RCC1 was localized on nuclear pores, but this localization failed in a temperature-sensitive mutant of Pim1/RCC1. The mutant cells exhibited hyper-condensed chromatin after mitosis due to prolonged association of condensin on the chromosomes. Conceivably, a condensin-dephosphorylation defect might cause hyper-condensed chromatin, since chromosomal localization of condensin is dependent on phosphorylation by cyclin-dependent kinase (CDK). Indeed, CDK-phospho-mimic mutation of condensin alone caused untimely condensin localization, resulting in hyper-condensed chromatin. Together, these results suggest that dephosphorylation of CDK sites of condensin might require the Ran-GTP gradient produced by nuclear pore-localized Pim1/RCC1.
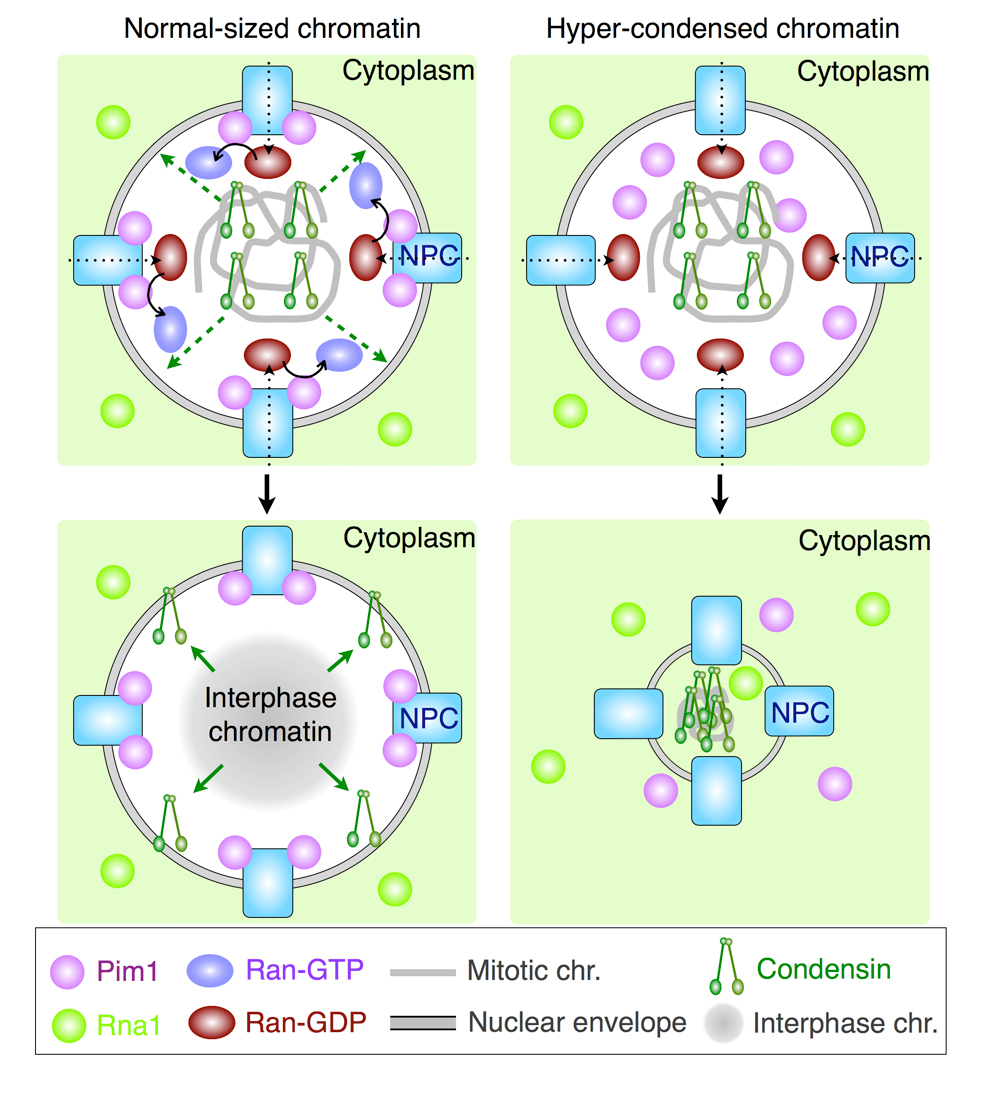
In a normal-sized nucleus (left), condensin is released from chromosomes in a Ran-GTP dependent manner. Therefore, a decondensed chromatin is formed in G1 phase. In a Pim1/RCC1-deficient nucleus, condensin is not released from chromosomes due to few Ran-GTP. Therefore, a hyper-condensed chromatin is formed in G1 phase.
A new function of rDNAs to compact bacterial chromosome
Microbial Genetics Laboratory / Niki Group
Multiple cis-acting rDNAs contribute to nucleoid separation and recruit the bacterial condensin, Smc-ScpAB
Koichi Yano, and Hironori Niki
Cell Reports, Vol. 21 Iss. 5, October 31, 2017 DOI:http://dx.doi.org/10.1016/j.celrep.2017.10.014
Condensins load onto DNA to organize chromosome. Smc-ScpAB clearly loads onto the parS sites bound by Spo0J, but other loading site(s) must operate independently of parS. In this study, we asked where and how Smc-ScpAB normally selects its loading site. Our results suggest that rDNA is also a loading site. A pull-down assay revealed that Smc-ScpAB preferentially loads onto rDNA in the wild-type cell and even in a Δspo0J mutant, but not in a Δsmc mutant. Moreover, we showed that deletion mutants of rDNAs cause a defect in nucleoid separation, and at least two rDNAs near oriC are essential for separation. Full-length rDNA including promoters is required for loading and nucleoid separation. A synthetic defect by deletions of both rDNA and spo0J resulted in more aberrant nucleoid separation. We propose that a single-stranded segment DNA that is exposed at highly transcribed rRNA operons would become a target for Smc-ScpAB loading.
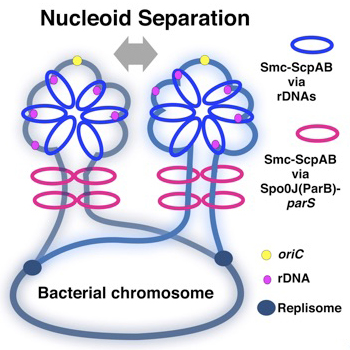
Schemes of compaction of newly replicated DNA strands. The yellow circle indicates oriC, black blue circles indicate replication forks, and magenta circles indicate rrn operons. The blue rings indicate Smc-ScpAB topologically loaded on rrn operons, and magenta rings represent Smc-ScpAB topologically loaded by ParB bound to parS as shown in Wang et al. (2017). In addition to separation of oriC, chromosome compaction would always be accompanied by proper chromosome segregation. The chromosomal region adjacent to oriC is packed by Smc in the WT so that the nucleoids are clearly separated.
Combination of multiple Shh enhancers controls tooth development in mouse
Mammalian Genetics Laboratory / Shiroishi Group
SHH signaling directed by two oral epithelium-specific enhancers controls tooth and oral development
Tomoko Sagai, Takanori Amano, Akiteru Maeno, Hiroshi Kiyonari, Hyejin Seo, Sung-Won Cho and Toshihiko Shiroishi
Scientific Reports, 7, Article number: 13004 (2017) DOI:10.1038/s41598-017-12532-y
Interaction between the epithelium and mesenchyme coordinates patterning and differentiation of oral cavity structures including teeth, palatal rugae and tongue papillae. SHH is one of the key signaling molecules for this interaction. Epithelial expression of Shh in the tooth buds and tongue papillae is regulated by at least two enhancers, MRCS1 and MFCS4. However, it is unclear how the two enhancers cooperate to regulate Shh. Here, we found that simultaneous deletion of MRCS1 and MFCS4 results in the formation of a supernumerary tooth in front of the first molar. Since deletion of either single enhancer barely affects tooth development, MRCS1 and MFCS4 evidently act in a redundant fashion. Binding motifs for WNT signaling mediators are shared by MRCS1 and MFCS4, and play a central role in regulating Shh expression, indicating that the two redundant enhancers additively exert their Shh regulation by responding to WNT signal input.
This study was carried out as a collaboration of Tomoko Sagai, Takanori Amano and Toshihiko Shiroishi of National Institute of Genetics, Hiroshi Kiyonari of RIKEN Center for Life Science Technologies, and Hyejin Seo and Sung-Won Cho of Yonsei University in Korea. This study was supported by JSPS KAKENHI 24247002.
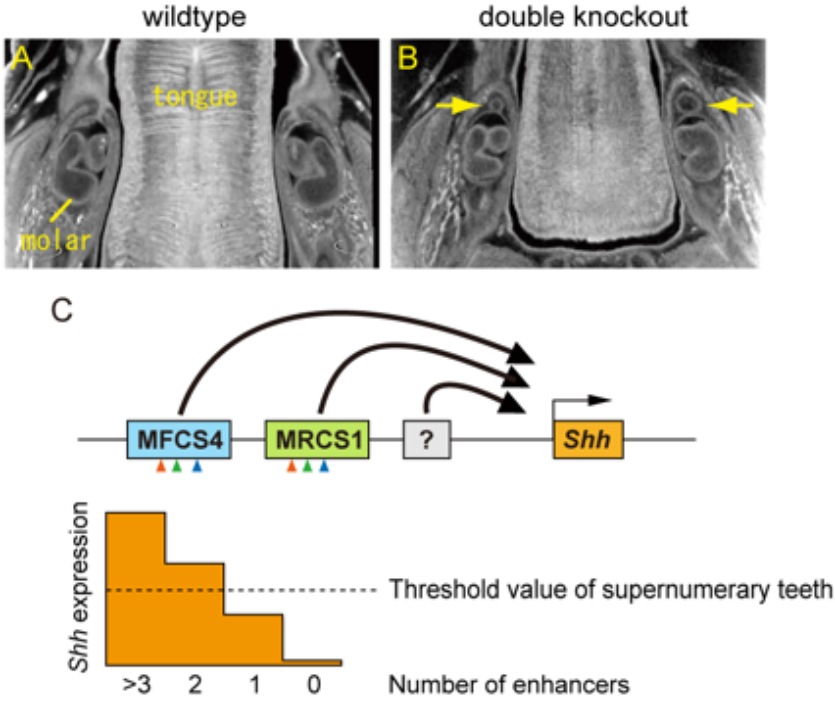
Supernumerary tooth formation in the mouse with simultaneous deletion (double knockout) of the MRCS1 and MFCS4 enhancers. (A and B) X-ray micro-CT images of transverse section of mandible in the wild type (A) and the mouse with the combinatorial deletion of MRCS1 and MFCS4 (B). Yellow arrow indicates supernumerary tooth. (C) A model of redundant action of the Shh enhancers. MRCS1 and MFCS4 share three common regulatory motifs that are located in the same order (arrowheads) and have redundant roles for the Shh expression in tooth buds. Expression levels of Shh are additively regulated by MRCS1, MFCS4, and probably unidentified enhancers. When reduction of Shh expression level falls below a threshold, supernumerary molars arise. MRCS1 and MFCS4 control different expression domains of Shh in the oropharyngeal tissues in addition to the tooth bud. Thus, the two redundant enhancers may cooperatively regulate the tooth development, while they have undergone sub-functionalization.
Spinal RacGAP α-chimaerin is required to establish the midline barrier for proper corticospinal axon guidance
Press release
Acute inactivation of the replicative helicase in human cells triggers MCM8–9-dependent DNA synthesis
Toyoaki Natsume, Kohei Nishimura, Sheroy Minocherhomji, Rahul Bhowmick, Ian D. Hickson, Masato T. Kanemaki
Genes & Development DOI:10.1101/gad.297663.117
Pressrelease (In Japanese only)
Dr. Toyoaki Natsume and Prof. Masato Kanemaki at National Institute of Genetics, ROIS, together with the group led by Prof. Ian D. Hickson at University of Copenhagen, reported a new system to deal with failure in DNA replication. The finding was published in Genes & Development in advance of the print journal.
In cell proliferation, genomic DNA has to be precisely copied into two (DNA replication) before equal distribution to two daughter cells. For doing this, double-stranded DNA is unwound, the process of which is similar to open a zipper of your clothes (Figure 1A). Similar to pulling the ‘slider’ for opening a zipper, the replicative helicase known as MCM2–7 moves on DNA for unwinding double-stranded DNA. However, because human genomic DNA is very long (approx. 2 m per cell), it is challenging to entirely unwind the genomic DNA. Occasionally, the MCM2–7 helicase falls off from DNA when it encounters a roadblock (such as DNA damage). Because reloading of MCM2–7 is strictly inhibited during S phase (when cells carry out DNA replication), this might lead to incompletion of DNA replication, which causes the loss of genetic information from daughter cells unless the cells have a mechanism to deal with the problem.
In this study, the research groups observed how human cells responded to artificial removal of the MCM2–7 helicase by using the auxin-inducible degron (AID) technology that they had developed previously (the information about this technology is described here). They revealed that the MCM8–9 helicase, which is evolutionally related to the MCM2–7 helicase, promotes a non-canonical DNA synthesis as a backup system after removal of MCM2–7 (Figure 1B).
Many anticancer drugs kills cancer cells by inducing DNA lesions, which enhance removal of MCM2–7 from DNA during DNA replication. An inhibitor of the MCM8–9 helicase might enhance the effect of existing anticancer drugs by shutting off this backup system (Figure 2).
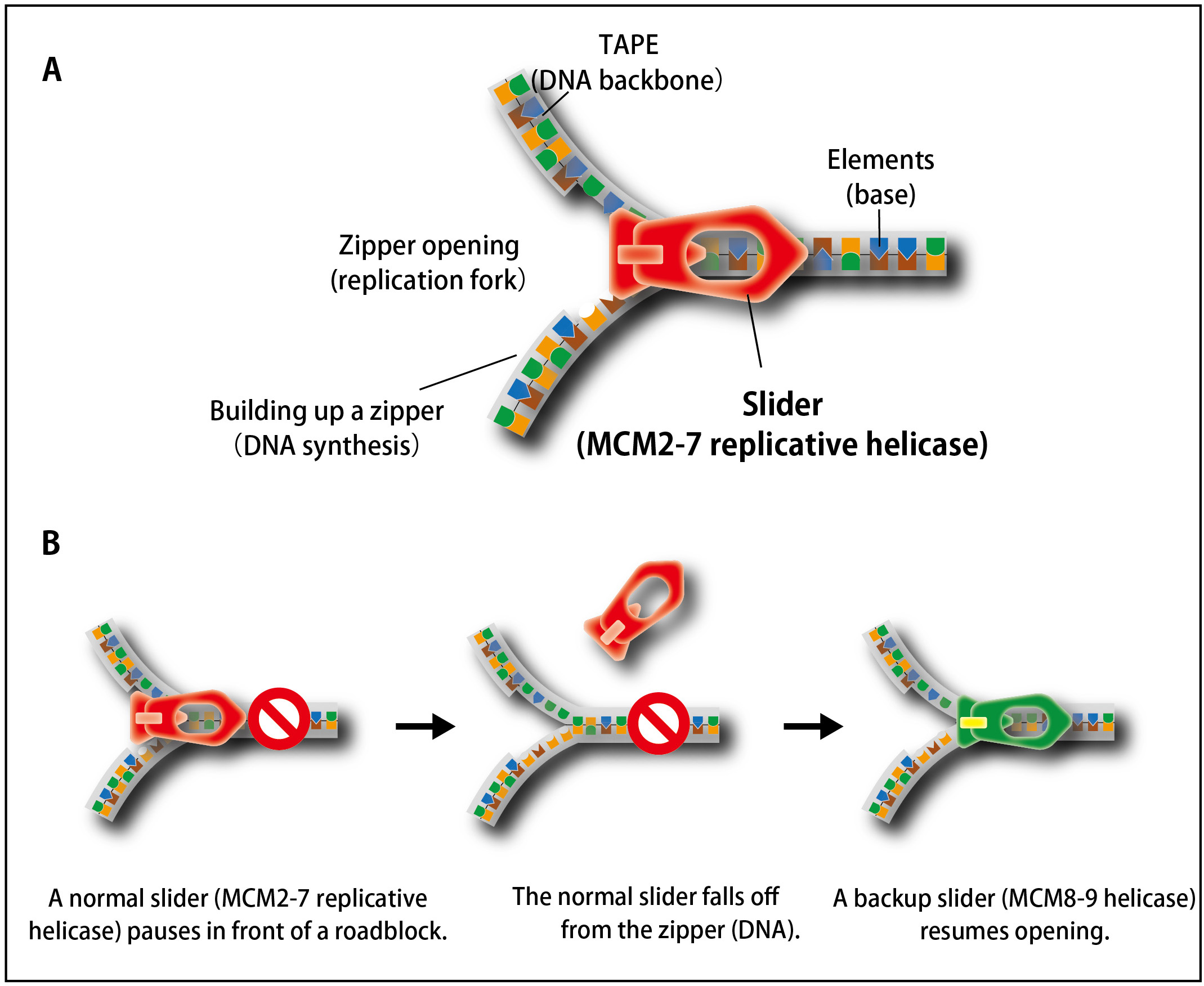
Figure 1. The process of unwinding double-stranded DNA during DNA replication is similar to that of opening a zipper of your clothes.
(A) Similar to the slider that opens a zipper, the MCM2–7 replicative helicase opens double-stranded DNA in cells.(B) When the replicative MCM2–7 helicase (a normal slider) encounters to a roadblock, it occasionally falls off from DNA. To continue DNA synthesis, cells recruit the MCM8–9 helicase as a backup slider.
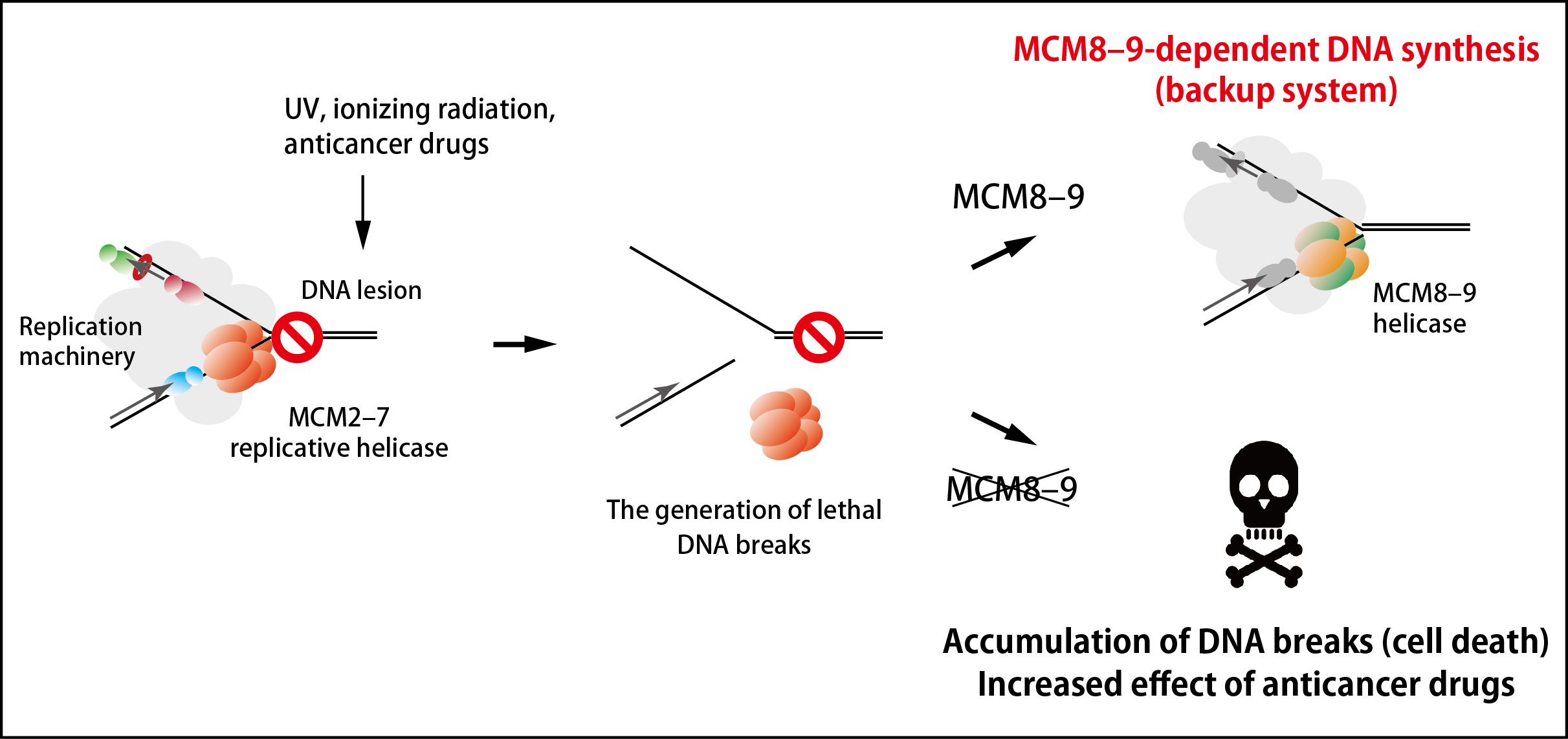
Figure 2. An MCM8–9-dependent backup system against failure in DNA replication.
The MCM2–7 replicative helicase falls off when encountered to a roadblock on DNA, leading to generation of DNA breaks. In this study, the research groups found that the MCM8–9 helicase continues DNA synthesis on behalf of the MCM2–7 (top right). If MCM8–9 does not work, the accumulation of DNA breaks results in cell death (bottom right).
Combination of multiple Shh enhancers controls tooth development in mouse
Mammalian Genetics Laboratory / Shiroishi Group
SHH signaling directed by two oral epithelium-specific enhancers controls tooth and oral development
Tomoko Sagai, Takanori Amano, Akiteru Maeno, Hiroshi Kiyonari, Hyejin Seo, Sung-Won Cho and Toshihiko Shiroishi
Scientific Reports, 7, Article number: 13004 (2017) DOI:10.1038/s41598-017-12532-y
Interaction between the epithelium and mesenchyme coordinates patterning and differentiation of oral cavity structures including teeth, palatal rugae and tongue papillae. SHH is one of the key signaling molecules for this interaction. Epithelial expression of Shh in the tooth buds and tongue papillae is regulated by at least two enhancers, MRCS1 and MFCS4. However, it is unclear how the two enhancers cooperate to regulate Shh. Here, we found that simultaneous deletion of MRCS1 and MFCS4 results in the formation of a supernumerary tooth in front of the first molar. Since deletion of either single enhancer barely affects tooth development, MRCS1 and MFCS4 evidently act in a redundant fashion. Binding motifs for WNT signaling mediators are shared by MRCS1 and MFCS4, and play a central role in regulating Shh expression, indicating that the two redundant enhancers additively exert their Shh regulation by responding to WNT signal input.
This study was carried out as a collaboration of Tomoko Sagai, Takanori Amano and Toshihiko Shiroishi of National Institute of Genetics, Hiroshi Kiyonari of RIKEN Center for Life Science Technologies, and Hyejin Seo and Sung-Won Cho of Yonsei University in Korea. This study was supported by JSPS KAKENHI 24247002.

Supernumerary tooth formation in the mouse with simultaneous deletion (double knockout) of the MRCS1 and MFCS4 enhancers. (A and B) X-ray micro-CT images of transverse section of mandible in the wild type (A) and the mouse with the combinatorial deletion of MRCS1 and MFCS4 (B). Yellow arrow indicates supernumerary tooth. (C) A model of redundant action of the Shh enhancers. MRCS1 and MFCS4 share three common regulatory motifs that are located in the same order (arrowheads) and have redundant roles for the Shh expression in tooth buds. Expression levels of Shh are additively regulated by MRCS1, MFCS4, and probably unidentified enhancers. When reduction of Shh expression level falls below a threshold, supernumerary molars arise. MRCS1 and MFCS4 control different expression domains of Shh in the oropharyngeal tissues in addition to the tooth bud. Thus, the two redundant enhancers may cooperatively regulate the tooth development, while they have undergone sub-functionalization.
Insights into land plant evolution garnered from the Marchantia polymorpha genome
Guideline for Application for 2018 NIG-JOINT (Joint Research and Research Meeting)(Application deadline: midnight (12:00am) on December 18th, 2017)
Message From NIGINTERN 2017
Message From NIGINTERN 2017
Mr. Harsh Nagpal (Division of Microbial Genetics) received Morishima Award
On September 8, 2017 Mr. Harsh Nagpal (Division of Microbial Genetics) received Morishima Award from the Department of Genetics, SOKENDAI. The Morishima Award is given to students in the Department of Genetics to honor their outstanding performances during PhD studies and to encourage further achievements.


* Pioneers in Genetics MORISHIMA, Hiroko















