Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Mechanism for synchronization of the chloroplast division with the eukaryotic cell cycle
Division of Symbiosis and Cell Evolution / Miyagishima Group
Chloroplast division checkpoint in eukaryotic algae.
Nobuko Sumiya, Takayuki Fujiwara, Atsuko Era, and Shin-ya Miyagishima
Proc. Natl. Acad. Sci. USA. (2016). Published online, DOI:10.1073/pnas.1612872113
Chloroplasts arose from a cyanobacterial endosymbiont, which introduced photosynthesis into eukaryotes. It is widely believed that synchronization of division in the eukaryotic host cell and in the endosymbiont was critical for the host cell to maintain the endosymbiont/chloroplast permanently. However, it is unclear how the division of the endosymbiont (the chloroplast) and host cell became synchronized. Using the unicellular red alga Cyanidioschyzon merolae, we show that the host cell enters into the metaphase only when chloroplast division has commenced. A similar phenomenon also was observed in the glaucophyte alga Cyanophora paradoxa. It thus seems likely that the acquisition of the cell-cycle checkpoint of chloroplast division played an important role in the establishment of the chloroplast in ancient algae.
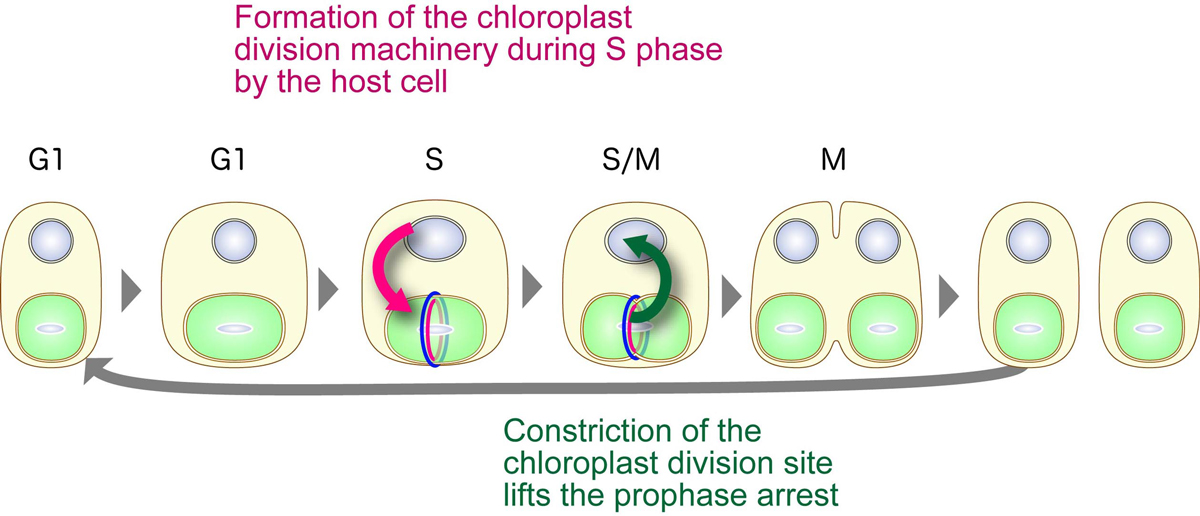
The interactive synchronization of division in the eukaryotic host cell and the chloroplast in algae. The host cell restricts the onset of chloroplast division to the S phase by S-phase–specific expression of nucleus-encoded chloroplast-division proteins. The formation of the competent chloroplast-division machinery and constriction of the chloroplast division site lift the prophase arrest so that the host cell enters into the metaphase only when chloroplast division progresses.
Message from NIGINTEN: 2016
Dynamic nucleosome movement provides information on chromatin structure
![]()
Dynamic nucleosome movement provides structural information of topological chromatin domains in living human cells
Soya Shinkai, Tadasu Nozaki, Kazuhiro Maeshima, Yuichi Togashi
PLOS Computational Biology. 12(10), e1005136 DOI:10.1371/journal.pcbi.1005136
Pressrelease (In Japanese only)
The mammalian genome is organized into submegabase-sized chromatin domains (CDs) including topologically associating domains, which have been identified using chromosome conformation capture-based methods. Single-nucleosome imaging in living mammalian cells has revealed subdiffusively dynamic nucleosome movement (left in Fig.). It is unclear how single nucleosomes within CDs fluctuate and how the CD structure reflects the nucleosome movement. Here, we present a polymer model wherein CDs are characterized by fractal dimensions and the nucleosome fibers fluctuate in a viscoelastic medium with memory. We analytically show that the mean-squared displacement (MSD) of nucleosome fluctuations within CDs is subdiffusive. The diffusion coefficient and the subdiffusive exponent depend on the structural information of CDs. This analytical result enabled us to extract information from the single-nucleosome imaging data for HeLa cells (right in Fig.). Our observation that the MSD is lower at the nuclear periphery region than the interior region indicates that CDs in the heterochromatin-rich nuclear periphery region are more compact than those in the euchromatin-rich interior region with respect to the fractal dimensions as well as the size. Finally, we evaluated that the average size of CDs is in the range of 100–500 nm and that the relaxation time of nucleosome movement within CDs is a few seconds. Our results provide physical and dynamic insights into the genome architecture in living cells.
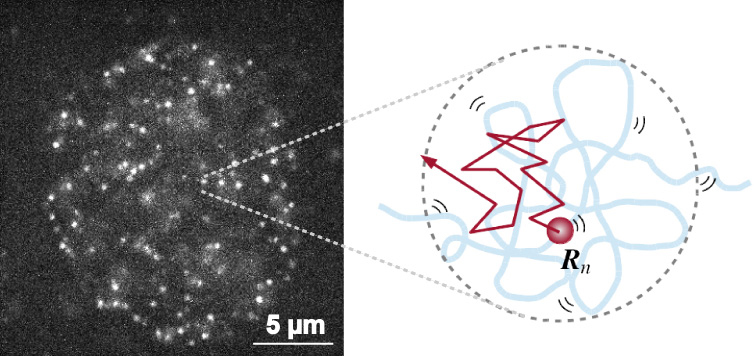
Single-nucleosome image in a living human nucleus (left). Nucleosome dynamically fluctuates within the chromatin domain and shows subdiffusion (right). By applying the mathematical model to the nucleosome movement, we can obtain the information on chromatin structure.
Reappointment of Director-General, National Institute of Genetics
The Research Organization of Information and Systems announced the ROIS Education and Research Council approved the reappointment of Dr. Isao Katsura who will expire his term of office on 30th of November, 2016 to the office of Director General, National Institute of Genetics.
His reappointment is effective from 1st of December, 2016 for a two year term.
Identification of feminizing factors for mouse germ cells
![]()
Sexual Fate Change of XX Germ Cells Caused by the Deletion of SMAD4 and STRA8 Independent of Somatic Sex Reprogramming
Quan Wu, Kurumi Fukuda, Yuzuru Kato, Zhi Zhou, Chu-Xia Deng, Yumiko Saga
PLOS Biology September 8, 2016 DOI:10.1371/journal.pbio.1002553
Pressrelease (In Japanese only)
Mammalian sex depends on a male-specific gene, sex-determining region Y (SRY), which is located on the Y chromosome. Individuals lacking this gene will develop as female. Accordingly, germ cell fate also changes from male to female in the absence of SRY. Therefore, it is thought that somatic cells regulate germ cells to become sperm or oocytes. However, it is largely unknown what factor is responsible for sexual fate determination in germ cells. In fetal ovaries, retinoic acid (RA) initiates STRA8 expression in germ cells and induces meiosis. Female germ cells without STRA8 fail to enter meiosis but still progress to oogenesis and form oocyte-like cells, indicating that RA is not the regulator of oogenesis. Here, we found that female germ cells lacking both SMAD4 and STRA8 (but not a single knockout) develop as male gonocyte-like cells in ovaries, indicating that these two factors work as female germ cell determinants. To our surprise, the sexual fate switch observed in the double knockout ovary is not accompanied by gene expression changes in somatic cells, revealing the unexpected finding that somatic factors controlled by SRY are dispensable for the upregulation of male-specific genes in germ cells. This research is partly supported by Grant-in-Aid for Scientific Research on Innovative Areas ”Epigenome dynamics and regulation in germ cells” to YS.
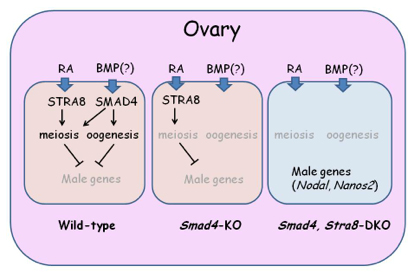
In the ovary, two pathways, RA-Stra8 and SMAD4 pathways play crucial roles. In the absence of SMAD4, germ cells fail to produce any oocytes but do not show any male character. Loss of SMAD4 and STRA8 leads to the upregulation of NANOS2 as well as other male-specific genes.
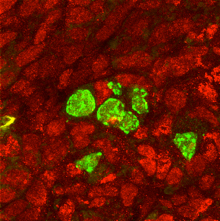
Smad4/Stra8-dKO germ cells express a male factor PLZF (green) in the ovary filled with female somatic cells (marked by Foxl2)。
Rice Argonaute protein required for reprogramming of histone H3 modifications on meiotic chromosomes.
Experimental Farm / Nonomura Group
Histone H3 modifications are widely reprogrammed during male meiosis I in rice dependently on MEL1 Argonaute protein
Hua Liu, Ken-Ichi Nonomura
Journal of Cell Science, Published online, 12 August, 2016 DOI:10.1242/jcs.184937
Meiosis is a special type of cell division to halve the chromosome number, achieved by two continuous division not intervened by DNA replication. It is an indispensable mechanism to generate genetic diversity via homologous chromosome pairing and meiotic recombination, in addition to stable transmission of genetic information to the next generation.
We focused on the relation of meiosis and histone modifications (glossary), which are important for control of chromosome structure and gene expression. Generally in plants, dimethylation at the position-9 lysine of histone H3 (H3K9me2) is thought to repress gene expressions and promote the compaction of chromatin structure. In contrast, acethylation at the same position (H3K9ac) activates gene expressions. We found that H3 modifications after the meiotic entry were totally altered from the premeiotic H3 status (Fig. 1A). “Large-scale meiotic chromosome reprogramming (LMR)” named in this paper is thought to be one of the mechanisms promoting meiosis in plants.
Interestingly, LMR was completely disrupted in the mutant of MEL1 (Fig. 1B), that is an Argonaute protein (glossary) specifically expressed in rice germ cells. These results suggest possibilities that MEL1 promotes meiosis via control of LMR, and that the RNA silencing mechanism is important for plant meiosis.
This work was supported by JSPS KAKENHI (25252004), and by NIG postdoc fellowship.
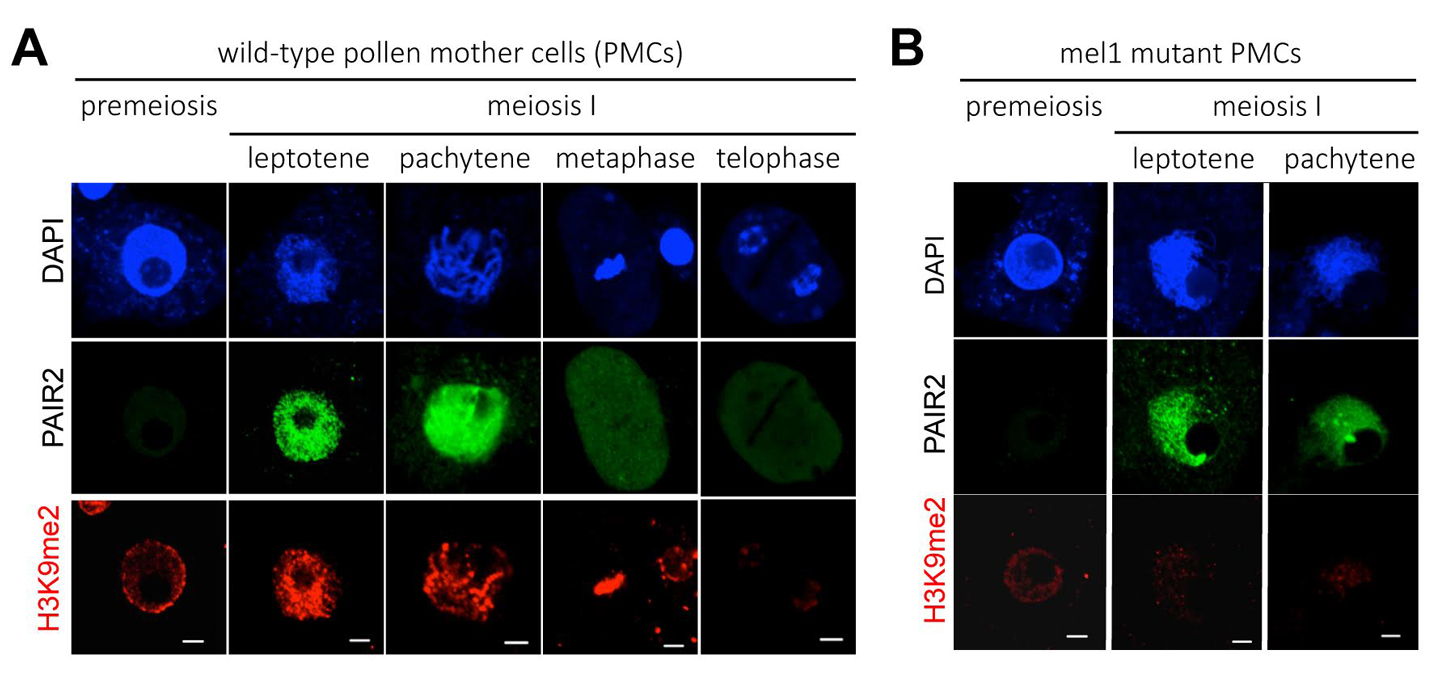
A wide reprogramming of histone H3K9me2 during meiosis I is dependent on the rice Argonaute protein MEL1.
(A) In wild-type pollen mother cells (PMCs), the level of H3K9me2 (red) is increased remarkably when cells transit from premeiosis to meiosis. Chromatin DNA is stained with DAPI (blue). PAIR2 (green) is a meiotic gene required for homologous chromosome pairing, and shows these cells undergo meiosis I. Scale bar = 5µm.
(B) mel1 mutant PMCs. PAIR2 signal (green) indicates these cells undergoing meiosis I, but no H3K9me2 reprogramming takes place.
<Glossary>
- Histone modification
- Chromatin, a structural element of chromosomes in eukaryotes, is composed of repetitive structure of nucleosomes including DNA and histone octamer (H2A, H2B, H3, H4). The N-terminal tail of histones protrudes from the DNA/histone core region. The amino acids of histone tails get various modifications, such as acetylation, methylation and phosphorylation. These modifications affect nucleosome states, resulting in alteration of gene expression and chromatin structure.
- Argonaute protein
- Argonaute (AGO) family proteins are highly conserved in eukaryotes. AGOs, in association with 20-30 nucleotides non-coding small RNAs, modulate both transcriptional and post-transcriptional gene silencing during various developmental events.
Estimation of driving forces for cytoplasmic streaming using data assimilation
Cell Architecture Laboratory / Kimura Group
Bayesian Inference of Forces Causing Cytoplasmic Streaming in Caenorhabditis elegans Embryos and Mouse Oocytes.
Niwayama R., Nagao H., Kitajima T. S., Hufnagel L., Shinohara K., Higuchi T., Ishikawa T., Kimura A.
PLoS ONE, Vol 11, e0159917 (2016). DOI:10.1371/journal.pone.0159917
Cytoplasmic streaming is observed in wide variety of cells both in animals and plants. It is caused in many cases by cytoskeletons and molecular motors. Where these forces are exerted is difficult to identify. In this study, we developed a novel computational method to estimate the localization and amplitude of the forces generating cytoplasmic streaming. Our method infers the distribution of forces by fitting the flow field in hydrodynamics simulation to that observed in cells. We applied the method to Caenorhabditis elegans embryos and mouse oocytes. The distinct patterns of force distribution estimated in this study were consistent with the proposed distinct functions of the streaming in both of these species. We expect our method to have diverse applications, and to serve as a powerful tool for biologists who want to characterize the mechanics of biological hydrodynamic flows.
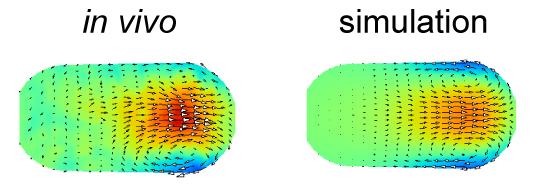
The velocity distribution in the simulation performed using the force distribution estimated in this study (right) agrees well with that measured experimentally (left). The color represents the velocity along the anterior-posterior axis, and the arrows represent the direction of the flow for cytoplasmic streaming in the C. elegans embryo.
Academic exchange agreement between NIG and Tokyo University of Agriculture
Also, the faculty of life science (tentative name) will be established in Tokyo University of Agriculture in FY2017. This faculty will further contribute to the collaboration.



Signing ceremony
New assistant professor joins NIG
New assistant professor joins NIG as of August 1, 2016.
Kazuo HARA: Laboratory for Gene-Expression Analysis, Okubo Group
Specific DNA-binding activity of bacterial condensin
Microbial Genetics Laboratory / Niki Group
In vitro topological loading of bacterial condensin MukB on DNA, preferentially single-stranded DNA rather than double-stranded DNA
Hironori Niki, and Koichi Yano
Scientific Reports 6, Article number: 29469 (2016) DOI:10.1038/srep29469
Condensin is the major driving force in the segregation of daughter chromosomes in prokaryotes. Core subunits of condensin belong to the SMC protein family, whose members are characterized by a unique ATPase activity and dimers with a V-shaped structure. The V-shaped dimers might close between head domains, forming a ring structure that can encircle DNA. Indeed, cohesin, which is a subfamily of SMC proteins, encircles double-stranded DNA to hold sister chromatids in eukaryotes. However, the question of whether or not condensin encircles the chromosomal DNA remains highly controversial. Here we report that MukB binds topologically to DNA in vitro, and this binding is preferentially single-stranded DNA (ssDNA) rather than double-stranded DNA. The binding of MukB to ssDNA does not require ATP. In fact, thermal energy enhances the binding. The non-SMC subunits MukF and MukE did stimulate the topological binding of MukB, although they hindered DNA-binding of MukB. Recent reports on the distribution of condensin in genomes reveal that actively transcribed genes in yeast and humans are enriched in condensin. In consideration of all these results, we propose that the binding specificity of condensin to chromosome is provided not by the DNA sequence but by the DNA structure, which is ssDNA.
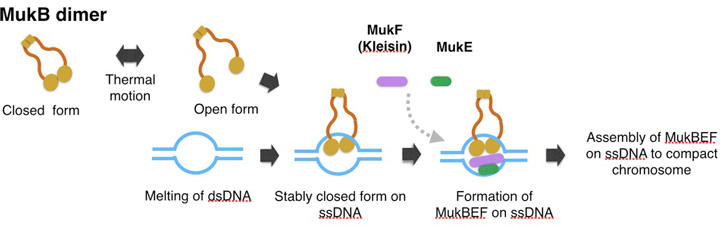
A model of topological binding of MukB in E. coli cells.
We hypothesize that the arms of MukB dimers are flexible and change between the open form or closed form depending on the thermal fluctuation. Occasionally the open form captures DNA, and then changes into the closed form. After the MukB dimer captures ssDNA, the closed form would become static because a part of the inner interface of a MukB globular domain interacts with ssDNA. Thus MukB would keep the captured DNA steady inside the ring of the dimer. Further, the MukB-DNA complex might be strengthened by MukEF, and then each of the MukBEF-DNA complexes would be assembled to compact chromosomal DNA. We infer that ATP hydrolysis is required for dissociation of the MukBEF-DNA complex from the assembled complexes.
A simple telomere labeling method in tissue sections.
Press release
Telomere Visualization in Tissue Sections using Pyrrole–Imidazole Polyamide Probes
Asuka Sasaki, Satoru Ide, Yusuke Kawamoto, Toshikazu Bando, Yukinori Murata, Mari Shimura, Kazuhiko Yamada, Akiyoshi Hirata, Kiyoshi Nokihara, Tatsumi Hirata, Hiroshi Sugiyama, Kazuhiro Maeshima
Scientific Reports 6: 29261 (2016) DOI:10.1038/srep29261
Press Release (Only in Japanese)
Pyrrole–Imidazole (PI) polyamides bind to specific DNA sequences in the minor groove with high affinity. Specific DNA labeling by PI polyamides does not require DNA denaturation with harsh treatments of heat and formamide and has the advantages of rapid and less disruptive processing. Previously, we developed tandem hairpin PI polyamide probes (TH59 series), which label telomeres in cultured cell lines more efficiently than conventional methods, such as fluorescence in situ hybridization (FISH). Here, we demonstrate that a TH59 derivative, HPTH59-b, along with immunostaining for specifying cell types in the tissues, visualizes telomeres in mouse and human tissue sections. Quantitative measurements of telomere length with single-cell resolution suggested shorter telomeres in the proliferating cell fractions of tumor than in non-tumor tissues. Thus, PI polyamides are a promising alternative for telomere labeling in clinical research, as well as in cell biology.
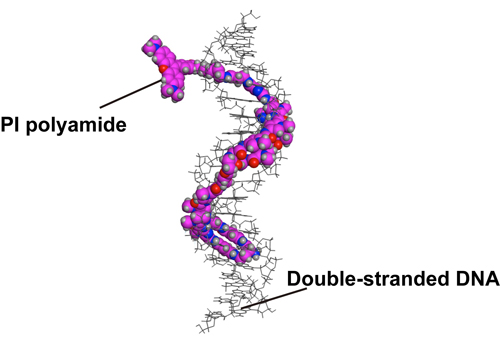
Figure1. A structural model of HPTH59-b binding to DNA.
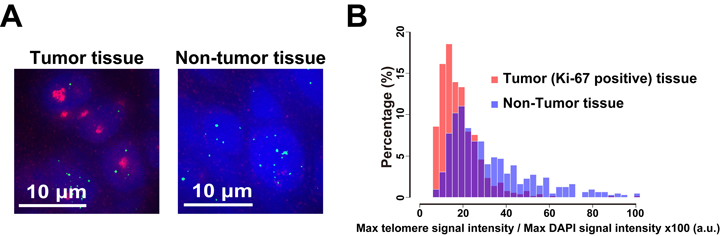
Figure2. Different telomere lengths between human tumor and non-tumor tissue sections. (A) Frozen sections of esophageal tumor/non-tumor tissue stained with DAPI (blue), anti-Ki-67 (growth marker; green) antibody, and HPTH59-b (red). Distribution histograms of telomere signal intensities in tumor and non-tumor tissue sections.
New assistant professor joins NIG
New assistant professor joins NIG as of July 1, 2016.
Daisuke TAKAO: Division of Centrosome Biology, Kitagawa Group
Emergence and evolution of Hominidae-specific coding and noncoding genomic sequences
Division of Population Genetics / Saitou Group
Emergence and evolution of Hominidae-specific coding and noncoding genomic sequences
Morteza Mahmoudi Saber, Isaac Adeyemi Babarinde, Nilmini Hettiarachchi and Naruya Saitou
Genome Biology and Evolution Volume 8, advance access, 2016 DOI:10.1093/gbe/evw132
Family Hominidae includes humans and great apes. We analyzed whole genome sequences to find Hominidae-specific genes and highly conserved noncoding sequences (HCNSs). We discovered that Down syndrome critical region 4 (DSCR4) is the only experimentally verified gene uniquely present in Hominidae. DSCR4 has no structural homology to any known protein and was inferred to have emerged in several steps. We also identified 1,658 Hominidae-specific HCNSs. These HCNSs were found to be under purifying selection, indicating that they may harbor important functions. They are in close proximity of genes involved in sensory perception of sound and developmental process, and also showed a significantly lower nucleosome occupancy probability. Interestingly, many ancestral sequences of the Hominidae-specific HCNSs showed very high evolutionary rates. This suggests that new functions emerged through some kind of positive selection, and then purifying selection started to operate to keep these functions.
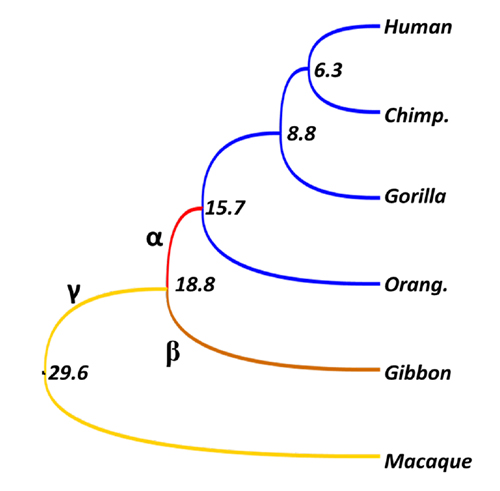
Evolutionary rates of each branch of Hominidae and their outgroups. Values are divergence times (million years). Although within-Hominidae rate is zero (identical sequences), rates for branches α, β, and γ 5.5, 2.0, and 1.9, respectively. In particular, the rate of branch α, which is common ancestor of Hominidae, is more than five times higher than neutral rate (1.0), and at least some ancestral sequences seem to experience positive selection.
2-Day workshop on scientific presentation ~ based on the “NIG Method”
1-2 July, 2016 | National Institute of Genetics (NIG), Mishima Application Deadline: June 17th
Objectives and Outline
Oral presentation is an integral part of scientific research and serves as an opportunity to disseminate your research to the scientific community. However scientific presentation is not an oral version of your research paper; presentation transmits not only research accomplishments but also valuable information about the presenters themselves: the breadth of their research interests, logical and critical thinking skills, future directions, and personality. To meet these hidden needs of scientific presentation we have developed a new methodology of scientific presentation, based on our experience of making many research presentations as well as attending numerous seminars. This workshop introduces essential elements of this methodology, called the “NIG Method”. NIG Method is aimed not only at nurturing skills to get your message across, but also at improving the quality of your science itself.
This two-day workshop consists of lectures and a “masterclass”. In lectures (day 1), we will discuss how research presentation differs from lectures, and describe two key structural elements of scientific presentation: “key question” and “perspective frame”. We will then introduce several techniques to aid comprehension — i.e. techniques to demonstrate your intelligence to the audience.
Masterclass session (day 2) is an opportunity to put the theory into practice. Selected participants will make a 10-minute presentation in English to receive advice from instructors and other researchers.
All activities will be conducted in English; fluency in Japanese is not required for participation.
Instructors
- Tatsumi Hirata (professor, Division of Brain Function)
- Todd Gorman (English Specialist)
- Yasushi Hiromi (professor emeritus, SOKENDAI)
Program (tentative)
Friday, 1 July
| 13:30-14:00: | lecture 1 | “Essence of scientific presentation” |
| 14:00-15:00: | lecture 2 | “Structure of scientific presentation” |
| 15:15-16:15: | lecture 3 | “Various techniques in scientific presentation” |
| 16:30-19:30: | Mixer | (NIG Poster Workshop) |
Saturday, 2 July
| 9:00-11:30: | Presentation masterclass | |
| 11:30-12:00: | lecture 4 | “Humor in scientific presentation” |
Eligibility
Researchers (Graduate Student, Postdoc, Faculty) in natural sciences
Maximum number of participants: 50
How to Apply
Please email the information listed below ask-ord@nig.ac.jp with the subject line: NIGmethod2016.
- Name
- Affiliation
- Current position
- Current Lab
- Brief description of your research (2-3 sentences)
-
Would you like to make a 10-minute presentation in masterclass? (Yes/No)
If you wish to make a presentation on your research, please submit tentative title and abstract.
Fee
Participation free: None
Need-based Travel Grant Available
We will be offering need-based travel grant. This grant is based on the financial need and distance required for travel to attend the workshop. To be considered, you should be registered at the time of application. Please send the information listed below with your application mail. Applicants will be notified of the results of their application by email by 21 June.
- A brief budget for the workshop including your anticipated expenses (lodging, travel costs, etc.)
- Other funds and their sources (e.g. department, advisor’s grant, etc) you already have to partially cover your travel expenses.
- A brief explanation (2-3 sentences) about why you are attending and what you are most expecting to the Workshop.
If you have any questions, write to ask-ord@nig.ac.jp
A simple non-arbitrary method to quantify fluorescent images
Mammalian Development Laboratory / Saga Group
GBIQ: a non-arbitrary, non-biased method for quantification of fluorescent images
Youichirou Ninomiya*, Wei Zhao and Yumiko Saga*
Scientific Reports 6, Article number: 26454 (2016) DOI:10.1038/srep26454
(* corresponding authors)
Non-arbitrary and non-biased quantification of fluorescent images is an essential tool for the data-centric approach to biological systems. Typical application is high-content analysis, where various phenotypic changes in cellular components are measured from fluorescent image data. A standard protocol to detect cellular phenotypes is cell-segmentation, in which boundaries of cellular components, such as cell nucleus and plasma membrane, are first identified to define cell segments, then acquiring various phenotypic data of each segment. To achieve reliable outcome, cell-segmentation requires manual adjustments of many parameters; this requirement could hamper automated image processing in high-throughput workflow, whose quantification must be non-arbitrary and non-biased. As a practical alternative to the method, we developed GBIQ (Grid Based Image Quantification), which allows comparison of cellular information without identification of single cells. GBIQ divides an image with tiles of fixed size grids and records statistics of the grids with their location coordinates, minimizing arbitrary intervenes. GBIQ requires only one parameter (size of grid) to be set; nonetheless it robustly produces results suitable for further statistical evaluation. The simplicity of GBIQ allows it to be readily implemented in an automated high-throughput image analysis workflow. This work was supported by the Data Assimilation and Simulation Support Technologies project from the Research Organization of Information and Systems (ROIS) in Japan.
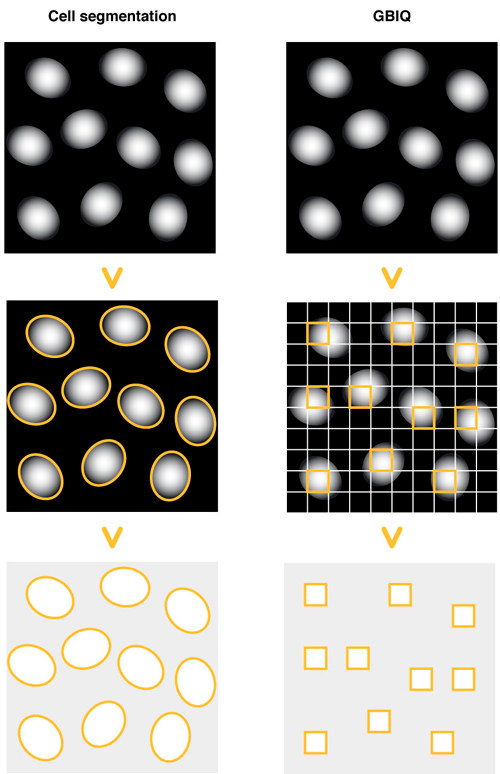
Cell segmentation: Draw outlines of cell nuclei using DNA counterstaining. Identify each outline of cell nucleus, then measure fluorescent feature of the outlines (segments).
GBIQ: Divide an image by tilling of fixed size grids. Filter certain grids that contain only cell nucleus, then measure fluorescent feature of the filtered grids.
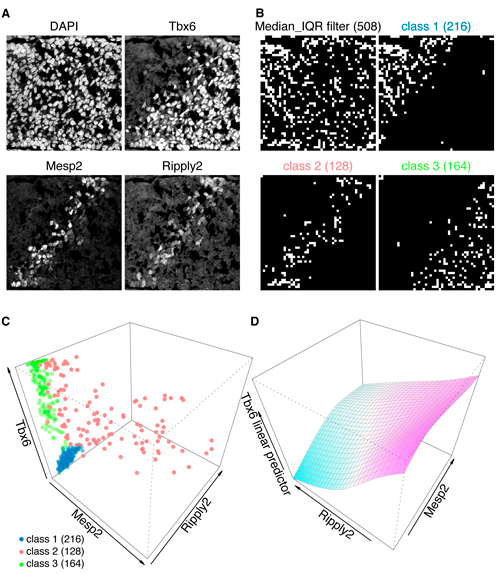
A: Triple immunofluorescent staining of developing tailbud from mouse embryo. Nuclear counterstaining by DAPI.
B and C: These immunofluorescent images (A) are processed by GBIQ with g=16. Applying the “Median_IQR filter” onto DAPI channel extracts reliable 508 observations (B, Median_IQR filter, white grids). Further applying Mclust utilizing the 3 factors (median intensities of Tbx6/Mesp2/Ripply2) onto the 508 dataset classifies them to 3 classes (C), which illustrate distinctive tissue architecture (B, class1, 2 and 3, white grids). C: All 3 factors are expressed in the class 2 (red), while neither Mesp2 nor Ripply2 expression is evident in the class 1 (blue) and class 3 (green).
D: Generalized additive model analysis of the class 2 dataset indicates negative correlation between Ripply2 and Tbx6, suggesting Ripply2 degrades Tbx6.
Notice regarding the English proficiency evaluation for the entrance examinations from FY2016
A novel antitumor platinum complex
Biological Macromolecules Laboratory / Maeshima Group
Chromatin folding and DNA replication inhibition mediated by a highly antitumor-active tetrazolato-bridged dinuclear platinum(II) complex
Ryosuke Imai, Seiji Komeda, Mari Shimura, Sachiko Tamura, Satoshi Matsuyama, Kohei Nishimura, Ryan Rogge, Akihiro Matsunaga, Ichiro Hiratani, Hideaki Takata, Masako Uemura, Yutaka Iida, Yuko Yoshikawa, Jeffrey C. Hansen, Kazuto Yamauchi, Masato T. Kanemaki, and Kazuhiro Maeshima
Scientific Reports 6, Article number: 24712 (2016) DOI:10.1038/srep24712
Chromatin DNA must be read out for various cellular functions, and copied for the next cell division. These processes are targets of many anticancer agents. Platinum-based drugs, such as cisplatin, have been used extensively in cancer chemotherapy. The drug–DNA interaction causes DNA crosslinks and subsequent cytotoxicity. Recently, it was reported that an azolato-bridged dinuclear platinum (II) complex, 5-H-Y, exhibits a different anticancer spectrum from cisplatin. Here, using an interdisciplinary approach, we reveal that the cytotoxic mechanism of 5-H-Y is distinct from that of cisplatin. 5-H-Y inhibits DNA replication and also RNA transcription, arresting cells in the S/G2 phase, and are effective to cisplatin-resistant cancer cells. Moreover, it causes much less DNA crosslinking than cisplatin, and induces chromatin folding. 5-H-Y will expand the clinical applications for the treatment of chemotherapy-insensitive cancers.
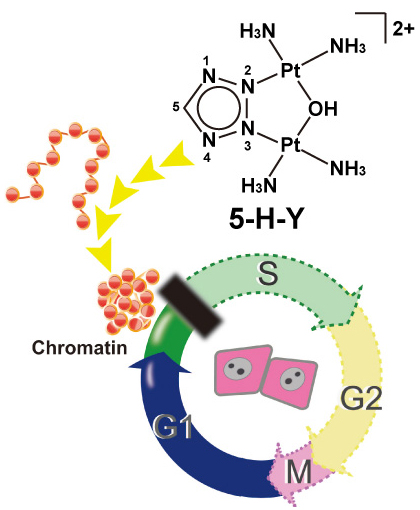
5-H-Y inhibits DNA replication and arrests the treated cells in S/G2 phase. 5-H-Y binds tightly to chromatin DNA and induces chromatin folding in vitro and in vivo.
Genomic locations of conserved noncoding sequences and relationship with their function
Division of Population Genetics / Saitou Group
Genomic locations of conserved noncoding sequences and their proximal protein-coding genes in mammalian expression dynamics
Isaac Adeyemi Babarinde and Naruya Saitou
Molecular Biology and Evolution DOI:10.1093/molbev/msw058
Conserved Noncoding Sequences (CNSs) in mammals were examined with special reference to their genomic location. We extracted the CNSs conserved between chicken and four mammalian species (human, mouse, dog and cattle). Human CNSs were confirmed to be under purifying selection. The distribution pattern, ChIP-Seq and RNA-Seq data suggested that the CNSs are regulatory elements. Physical distances between CNS and their nearest protein coding genes were well conserved between human and mouse genomes. ChIP-Seq signal and gene expression patterns also suggested that CNSs regulate nearby genes. Genes with more CNSs have more evolutionarily conserved expression than those with fewer CNSs. These results suggest that the genomic locations of CNSs are important for their regulatory functions. In fact, various kinds of evolutionary constraints may be acting to maintain the genomic locations of CNSs and protein-coding genes in mammals to ensure proper regulation. First author of this paper, Dr. Babarinde, just receive Ph.D. from Department of Genetics, SOKENDAI. He also received Morishima Award from NIG.
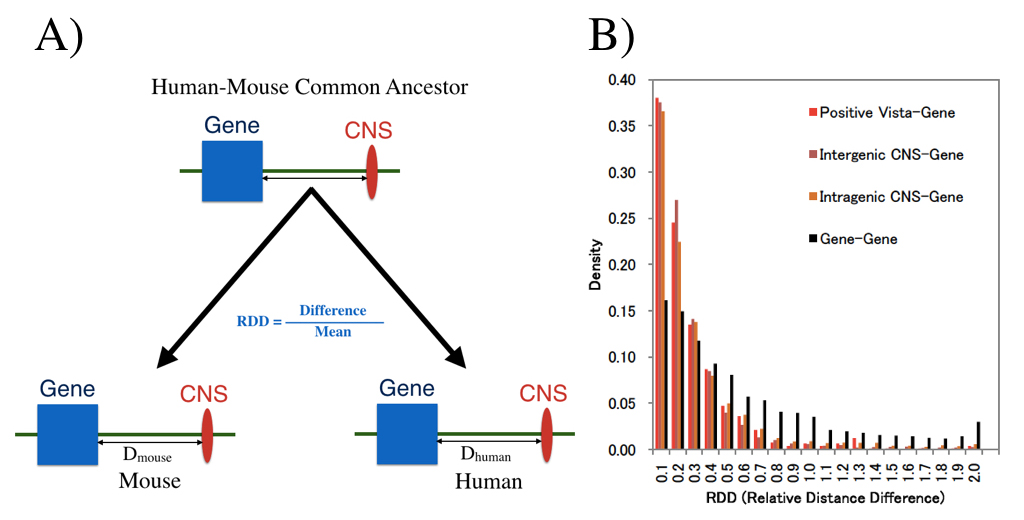
We examined whether genomic distances between genes and CNSs which already existed in the common ancestor of human and house are conserved now. (A) shows definition of distance measure RDD, and (B) shows RDD distribution. Distances between CNS and genes are much more conserved than those between genes.
















