Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
ROS production at micrometer-scale precision by receptor signaling
SCHENGEN receptor module drives localized ROS production and lignification in plant roots
Satoshi Fujita, Damien De Bellis, Kai H Edel, Philipp Köster, Tonni Grube Andersen, Emanuel Schmid-Siegert, Valérie Denervaud Tendon, Alexander Pfister, Peter Marhavý, Robertas Ursache, Verónica G. Doblas, Marie Barberon, Jean Daraspe, Audrey Creff, Gwyneth Ingram, Jörg Kudla, Niko Geldner
EMBO J (2020)e103894 DOI:10.15252/embj.2019103894
Reactive oxygen species (ROS) impact many physiological processes in animals or plants, but its production by NADPH oxidases is strictly regulated because of extremely high reactivity. Many plant receptor pathways are known as critical regulators of ROS production, but how they spatially control ROS production is yet to be clarified.
Fujita et al. (2020) established a phospho-signaling pathway that integrates direct, rapid activation of ROS production with positional information and transcriptional changes to form proper diffusion barriers. Firstly, the authors presented a direct connection from a peptide-receptor complex to NADPH oxidases via a membrane-anchored kinase by biochemically. Next, the authors focused on the membrane-anchored kinase that localizes on the plasma membrane in a polar fashion. Manipulation of the kinase localization successfully showed that this biased distribution of the kinase protein gave the positional cue, which enables the SCHENGEN pathway to activate only one side of the Casparian strip at micrometer-scale precision. This work would contribute to highlight how other receptor pathways could control local ROS production.
This work was mainly done by Dr. Satoshi Fujita (former postdoctoral fellow in University of Lausanne, currently NIG research fellow) in Prof. Niko Geldner’s group with a collaboration with the Central imaging, Electron microscope, and Genomics facilities of University of Lausanne, Swiss Institute of bioinformatics, Prof. Jörg Kudla’s group at University of Munster and Dr. Gwyneth Ingram’s group at University of Lyon.
This work was aided by grants no. 31003A_156261 and 310030E_176090 (N.G.), from the Swiss National Science Foundation, an ERC Consolidator Grant (616228-ENDOFUN) (N.G.), DFG grant (Ku931/14-1 (J.K.)) FEBS long-term fellowship (P.M.), EMBO long-term fellowship (R.U., M.B.), Marie Curie postdoctoral fellowship (T.G.A,), Fundacion Alfonso Martin Escudero fellowship (V.G.D.), and a Japan Society for the Promotion of Science (JSPS) fellowship (S.F.).
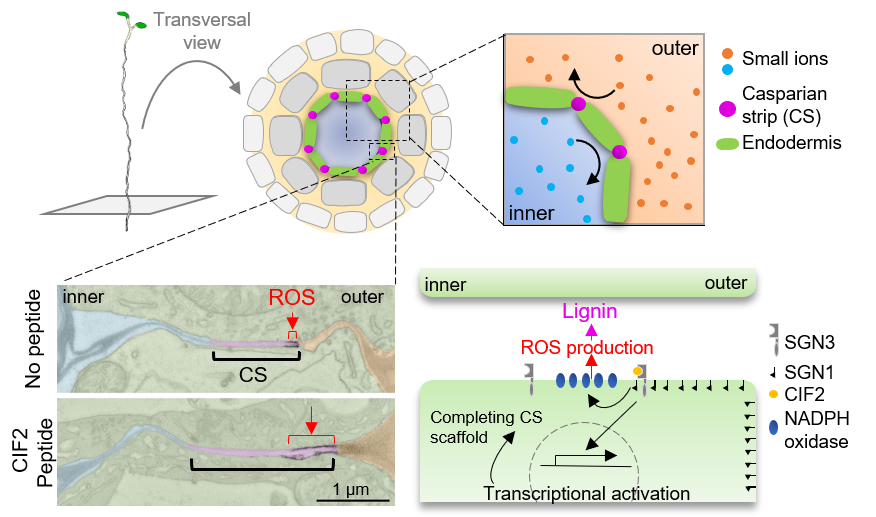
Figure: Plant roots have a lignin-based diffusion barrier, namely Casparian strips. Localized ROS production (EM pictures) by asymmetrical signal activation (schematic model) sustains functional barrier formation.
- RNA seq data(transcriptional changes after CIF peptide treatment)
Gastrointestinal Neurons Expressing HCN4 Regulate Retrograde Peristalsis
Gastrointestinal Neurons Expressing HCN4 Regulate Retrograde Peristalsis
Kensuke Fujii, Koichi Nakajo, Yoshihiro Egashira, Yasuhiro Yamamoto, Kazuya Kitada, Kohei Taniguchi, Masaru Kawai, Hideki Tomiyama, Koichi Kawakami, Kazuhisa Uchiyama, and Fumihito Ono
Cell Reports 30(9), 2879-2888 (2020). DOI:10.1016/j.celrep.2020.02.024
Peristalsis is indispensable for physiological function of the gut. The enteric nervous system (ENS) plays an important role in regulating peristalsis. While the neu- ral network regulating anterograde peristalsis, which migrates from the oral end to the anal end, is charac- terized to some extent, retrograde peristalsis re- mains unresolved with regards to its neural regula- tion. Using forward genetics in zebrafish, we reveal that a population of neurons expressing a hyperpo- larization-activated nucleotide-gated channel HCN4 specifically regulates retrograde peristalsis. When HCN4 channels are blocked by an HCN channel inhibitor or morpholinos blocking the protein ex- pression, retrograde peristalsis is specifically attenu- ated. Conversely, when HCN4(+) neurons expressing channelrhodopsin are activated by illumination, retrograde peristalsis is enhanced while anterograde peristalsis remains unchanged. We propose that HCN4(+) neurons in the ENS forward activating sig- nals toward the oral end and simultaneously stimu- late local circuits regulating the circular muscle.
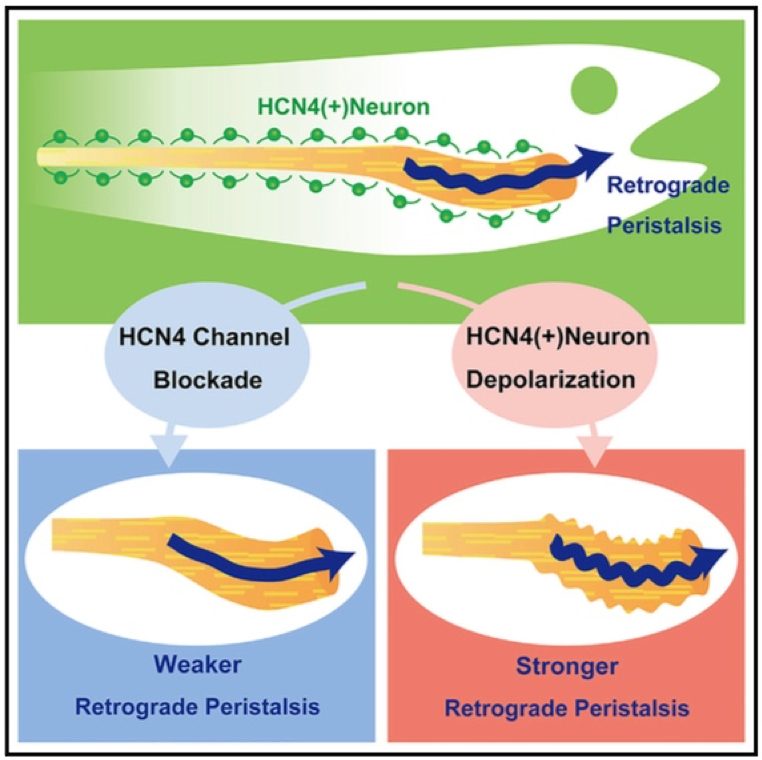
Figure1: Gastrointestinal neurons expressing a hyperpolarization-activated nucleotide- gated channel HCN4 regulate retrograde peristalsis, which migrates from the anal end to the oral end of the gut.
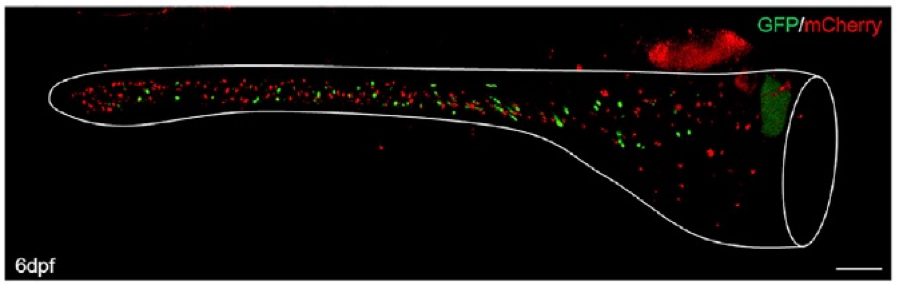
Figure2: Visualization of neurons (red) and HCN4-expressing neurons (green) in the zebrafish intestine.
A virtual reality system to analyze neural activity and behavior in adult zebrafish
Press release
A virtual reality system to analyze neural activity and behavior in adult zebrafish
Kuo-Hua Huang, Peter Rupprecht, Thomas Frank, Koichi Kawakami, Tewis Bouwmeester and Rainer W. Friedrich
Nature methods 02 March 2020 DOI:10.1038/s41592-020-0759-2
Press release (In Japanese only)
Virtual realities are powerful tools to analyze and manipulate interactions between animals and their environment and to enable measurements of neuronal activity during behavior. In many species, however, optical access to the brain and/or the behavioral repertoire are limited. We developed a high-resolution virtual reality for head-restrained adult zebrafish, which exhibit cognitive behaviors not shown by larvae. We noninvasively measured activity throughout the dorsal telencephalon by multiphoton calcium imaging. Fish in the virtual reality showed regular swimming patterns and were attracted to animations of conspecifics. Manipulations of visuo-motor feedback revealed neurons that responded selectively to the mismatch between the expected and the actual visual consequences of motor output. Such error signals were prominent in multiple telencephalic areas, consistent with models of predictive processing. A virtual reality system for adult zebrafish therefore provides opportunities to analyze neuronal processing mechanisms underlying higher brain functions including decision making, associative learning, and social interactions.
Source: Kuo-Hua Huang , et al., Published: 02 March 2020
DOI: 10.1038/s41592-020-0759-2
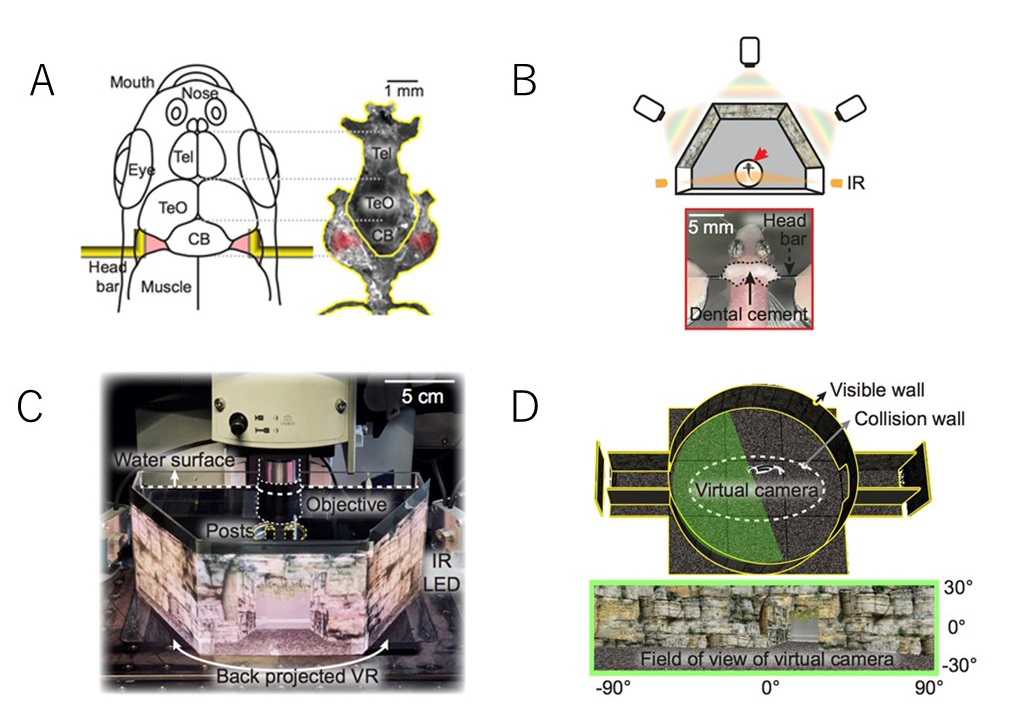
Fig: Virtual reality system to analyze neural activity and behavior in adult zebrafish
A: Head fixation with L-shaped bars
B: Virtual reality projected by three projectors onto a panoramic screen
C: Setup for virtual reality and two-photon imaging
D: Virtual reality seen by the head-fixed zebrafish
- This study is based on the previous study.
ALS mystery illuminated by blue light
Press release
Optogenetic modulation of TDP-43 oligomerization accelerates ALS-related pathologies in the spinal motor neurons
Kazuhide Asakawa, Hiroshi Handa, Koichi Kawakami
Nature Communications 11, 1004 (2020) DOI:10.1038/s41467-020-14815-x
Press release (In Japanese only)
A joint research group in Japan has succeeded in reproducing key ALS symptoms in a small tropical fish by remote controlling a disease-associated protein molecule using light illumination.
In amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease or motor neuron disease, nerve cells called motor neurons progressively degenerate. These motor neurons accumulate inclusions containing an aggregated form of TDP-43 protein.
In human body, motor neurons align along the spinal cord length and extend along the cables called axons to connect with muscles covering the body surface. This anatomical feature makes motor neurons one of the most difficult cells to observe. Consequently, we do not fully understand when and how healthy motor neurons begin to become abnormal and pathological in ALS. Read More>
Video: An optogenetic ALS zebrafish showing motor decline after blue light illumination (right).
EurekAlert!, the online, global news service operated by AAAS, the science society, PUBLIC RELEASE: 26-FEB-2020
- You can read the review here.
Appetite control via hunger and satiety
A bidirectional network for appetite control in zebrafish. Caroline Lei Wee
Erin Yue Song, Robert Evan Johnson, Deepak Ailani, Owen Randlett, Ji-Yoon Kim, Maxim Nikitchenko, Armin Bahl, Chao-Tsung Yang, Misha B Ahrens, Koichi Kawakami, Florian Engert, and Sam Kunes.
eLife 8:e43775 (2019). DOI:10.7554/eLife.43775
Medial and lateral hypothalamic loci are known to suppress and enhance appetite, respectively, but the dynamics and functional significance of their interaction have yet to be explored. Here we report that, in larval zebrafish, primarily serotonergic neurons of the ventromedial caudal hypothalamus (cH) become increasingly active during food deprivation, whereas activity in the lateral hypothalamus (LH) is reduced. Exposure to food sensory and consummatory cues reverses the activity patterns of these two nuclei, consistent with their representation of opposing internal hunger states. Baseline activity is restored as food-deprived animals return to satiety via voracious feeding. The antagonistic relationship and functional importance of cH and LH activity patterns were confirmed by targeted stimulation and ablation of cH neurons. Collectively, the data allow us to propose a model in which these hypothalamic nuclei regulate different phases of hunger and satiety and coordinate energy balance via antagonistic control of distinct behavioral outputs.
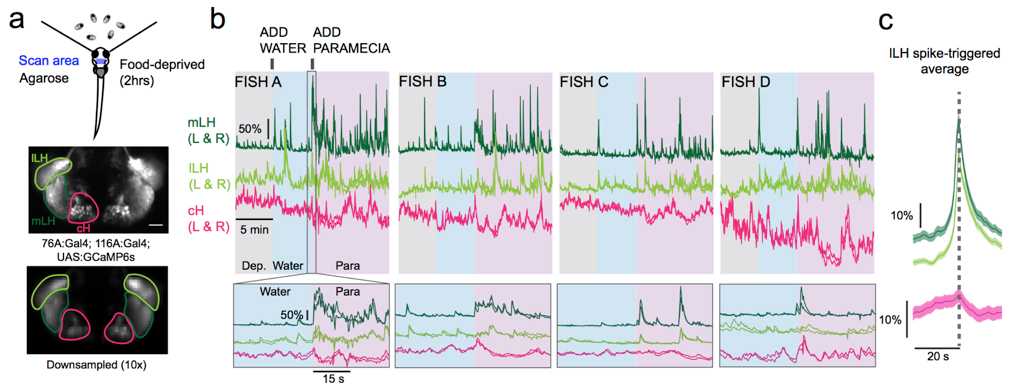
Figure: Calcium imaging of cH and LH by using transgenic zebrafish. By addition of paramecia (food), cH activity was reduced and LH activity was increased.
▶This study is based on the previous study.
Essential roles of autophagy in endosperm development in rice seed maturation
Essential roles of autophagy in metabolic regulation in endosperm development during rice seed maturation
Yuri Sera, Shigeru Hanamata, Shingo Sakamoto, Seijiro Ono, Kentaro Kaneko, Yuudai Mitsui, Tomoko Koyano, Naoko Fujita, Ai Sasou, Takehiro Masumura, Hikaru Saji, Ken-Ichi Nonomura, Nobutaka Mitsuda, Toshiaki Mitsui, Takamitsu Kurusu, Kazuyuki Kuchitsu
Scientific Reports 9, 18544 (2019) DOI:10.1038/s41598-019-54361-1
Autophagy, the recycling system of metabolites in eukaryotic cells, plays crucial roles in developmental processes, reproduction and biotic/abiotic-stress responses. However, the role in plants has been largely elusive.
We found that rice autophagy-deficient mutants set smaller seeds with chalky endosperms, in which starch granules were smaller and sparser than in the wild type (Figure). The activity of α-amylases, starch degradation enzymes, was abnormally activated in the mutant endosperm, and in contrast, the level of starch synthesis-promoting enzymes was reduced. The levels of heat shock-, oxidation- or high temperature-responsible proteins were elevated in the mutant endosperm. These results suggest the importance of autophagy in starch degradation and/or stress response pathways in rice endosperm development, and will be useful for breeding of high yielding varieties tolerant to environmental changes.
This work was achieved by collaboration of Tokyo University of Science, Suwa University of Science, Niigata University, National Institute of Advanced Industrial Science and Technology, National Institute of Genetics, Akita Prefectural University, Kyoto Prefectural University and National Institute for Environmental Studies, and was supported by NIG-JOINT (84A2018).
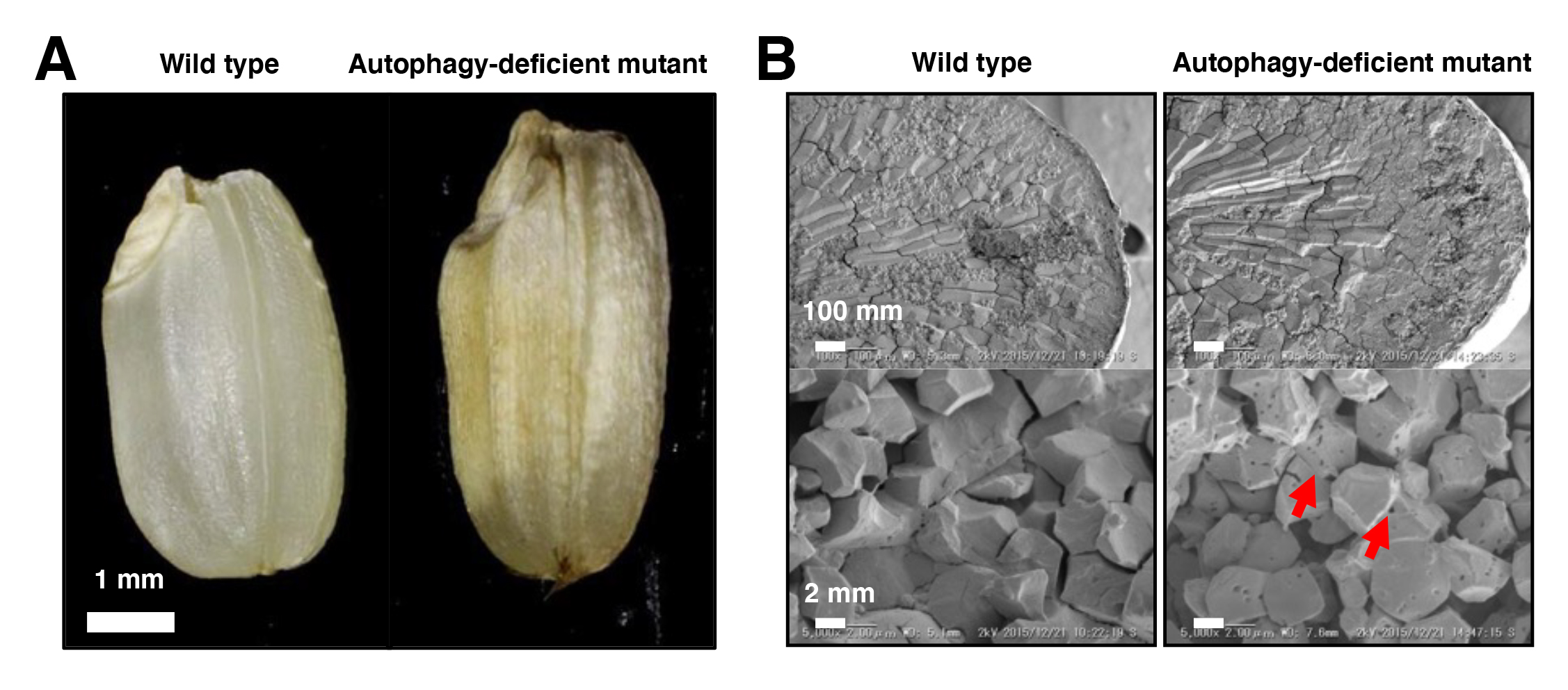
Figure: Mutant phenotypes in the endosperm of autophagy-deficient rice seeds
(A) The autophagy-deficient grain displays chalky appearance. (B) Scanning electron microscopic (top) and electron probe microscopic (bottom) images of endosperm sections. The starch granules had a lot of small pits in autophagy-deficient seeds (red arrows).
Structure-basis of signal ignition in spatial regulation of cell wall modification
Molecular mechanism for the recognition of sequence-divergent CIF peptides by the plant receptor kinases GSO1/SGN3 and GSO2
Satohiro Okuda*, Satoshi Fujita*, Andrea Moretti, Ulrich Hohmann, Verónica G. Doblas, Yan Ma, Alexandre Pfister, Benjamin Brandt, Niko Geldner, Michael Hothorn
*These authors are equally contributed to this work
Proceedings of the National Academy of Sciences PNAS first published January 21, 2020 DOI:10.1073/pnas.1911553117
Higher plants develop Casparian strips in their roots to restrict simple diffusion of small molecules in their extracellular spaces to maintain homeostasis of whole plant bodies. Previous studies showed that a pair of receptor-kinase, GSO1(SGN3), and 21a.a.-sulfated peptides, CIF1/2, were required for Casparian strip maturation, but detail molecular mechanisms remained unclear.
Okuda and Fujita et al. presented a crystal structure of CIF2- GSO1(SGN3) peptide-receptor complex at 3 Å resolution. Quantitative analysis revealed that the GSO1(SGN3) and CIF2 pair had one of the highest affinities among known peptide-receptor pairs. Structure-based prediction uncovered Ile81 of CIF2 as a critical residue to form a tri-complex with newly identified co-receptors, SERK family proteins, to activate the downstream of the signaling pathway. Moreover, the structure-guided analysis identified homologs of CIF1/2, namely CIF3/4. CIF3/4 directly binds to GSO1(SGN3) and its homolog GSO2, but their binding properties are different from CIF1/2. Additionally, neither CIF3 nor 4 are expressing in root endodermal cells, where Casparian strips are made, implying these newly identified peptides play roles in different contexts. Our biochemical and developmental approaches provide a molecular framework to understand the recognition of diverse peptide hormones in plants.
This work was aided by grants no. 31003A_156261 and 310030E_176090 (N.G.), 31003A_176237 and 31CP30_180213 (M.H.) from the Swiss National Science Foundation, an ERC Consolidator Grant (616228-ENDOFUN) (N.G.), and an International Research Scholar Award from the Howard Hughes Medical Institute (M.H.), a Human Frontier Science Program Organization (HFSPO) postdoctoral fellowship no. LT000567/2016-L (S.O.) and a Japan Society for the Promotion of Science (JSPS) fellowship (S.F.).
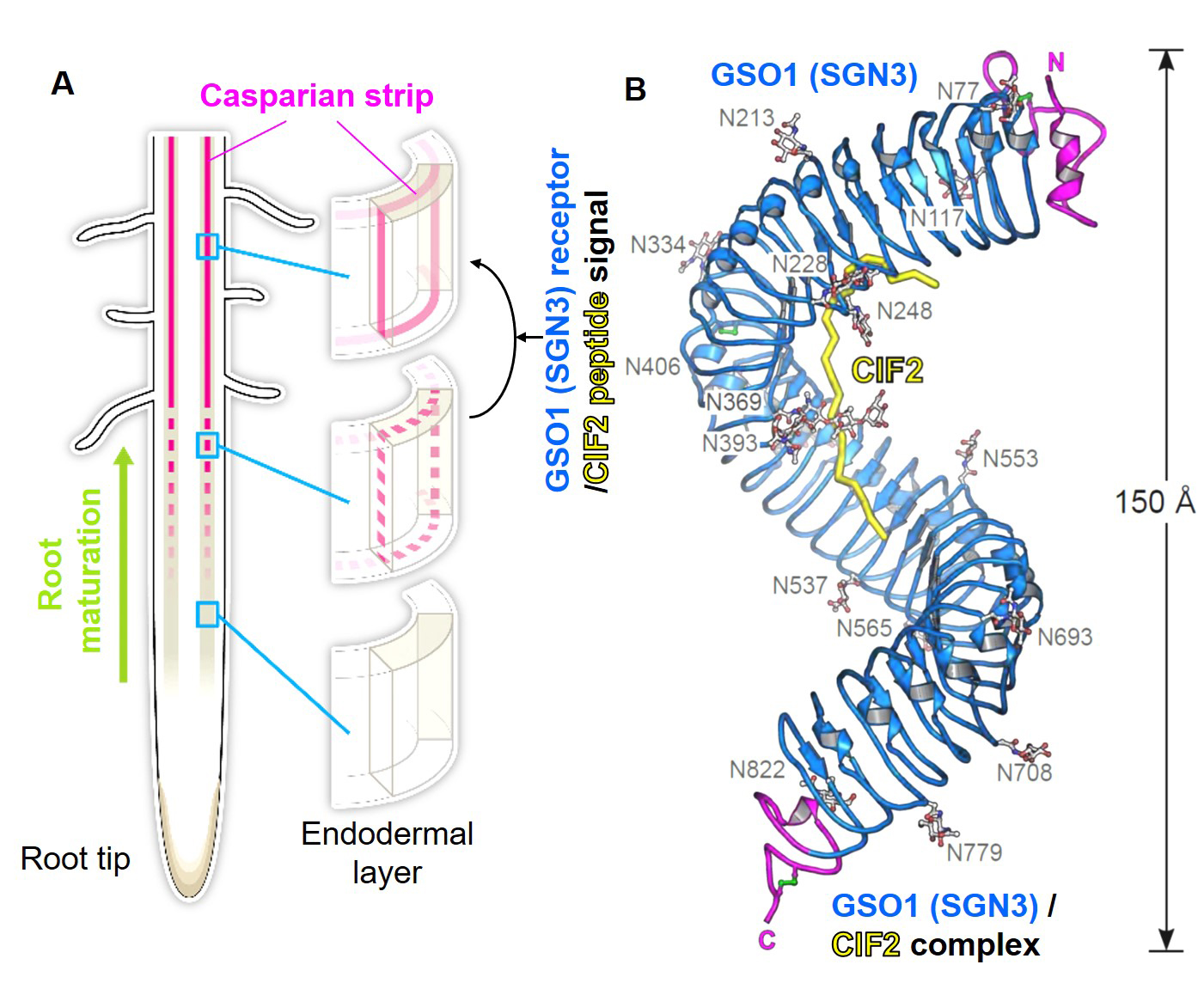
Figure: (A) Schematic model of Casparian strips (magenta). After establishing the connectivity (third row), this ring-like structure functions as an extracellular barrier. (Illustrated by Hiroko Uchida http://uchidahiroko.com/)
(B) Crystal structure of the GSO1(SGN3)/CIF2 receptor-peptide complex.
New assistant professor joins NIG
New assistant professor joins NIG as of January 1, 2020.
SASAKI, Takema : Oda Group • Cell Dynamics and Signaling Laboratory















