Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
How does the colony of Porpita porpita behave like a single organism?
Technical Section / Phenotype Research Center / Cell Architecture Laboratory
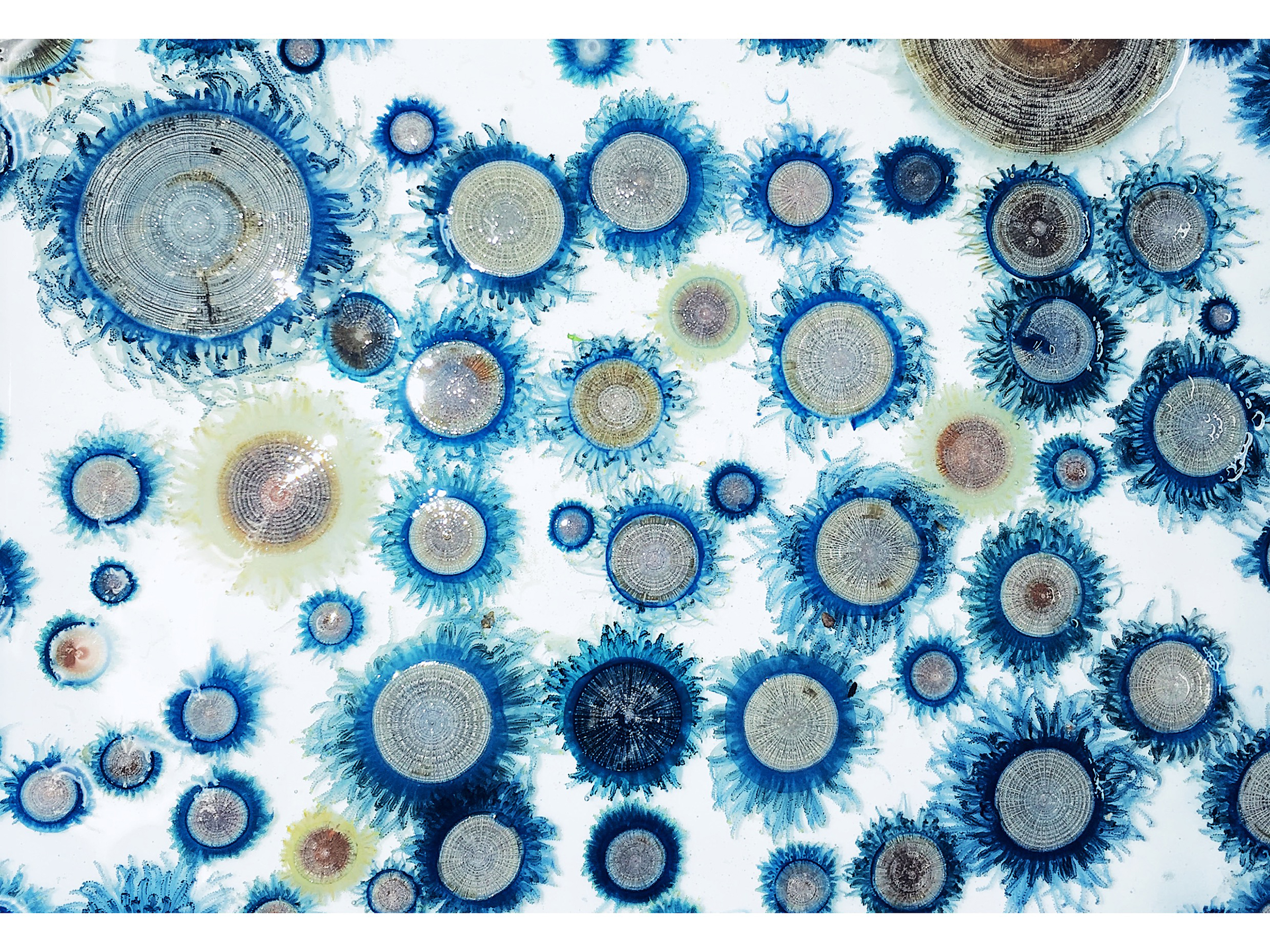
Zooid arrangement and colony growth in Porpita porpita
Kohei Oguchi, Akiteru Maeno, Keita Yoshida, Gaku Yamamoto, Hisanori Kohtsuka and Casey W. Dunn
Frontiers in Zoology (2025) 22, 11 1-12 DOI:10.1186/s12983-025-00565-3
The blue button Porpita porpita shows a highly integrated colony composed of functionally specialized zooids. Surface-floating organisms like P. porpita are called pleuston and form unique ecosystems in the ocean’s surface layers. However, due to their sensitivity to wind and currents, predicting their appearance is difficult, and their life history remains poorly understood. P. porpita is especially fragile and hard to maintain in captivity, making its colony development largely unknown. In this study, we analyzed specimens of various sizes collected from Sagami Bay, Japan, using histological sections and micro-CT. We found that each colony always had one central gastrozooid, and the number and size of gonozooids and dactylozooids increased with colony growth. Budding zones and the colony’s regenerative capacity suggest how such a superorganism is developed.
This work was supported by a NIG-JOINT (25A2020,70A2021) from National Institute of Genetics, Grant-in-Aid for Research Activity Start-up (No. 22K20662) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and a grant from the Research Institute of Marine Invertebrates.
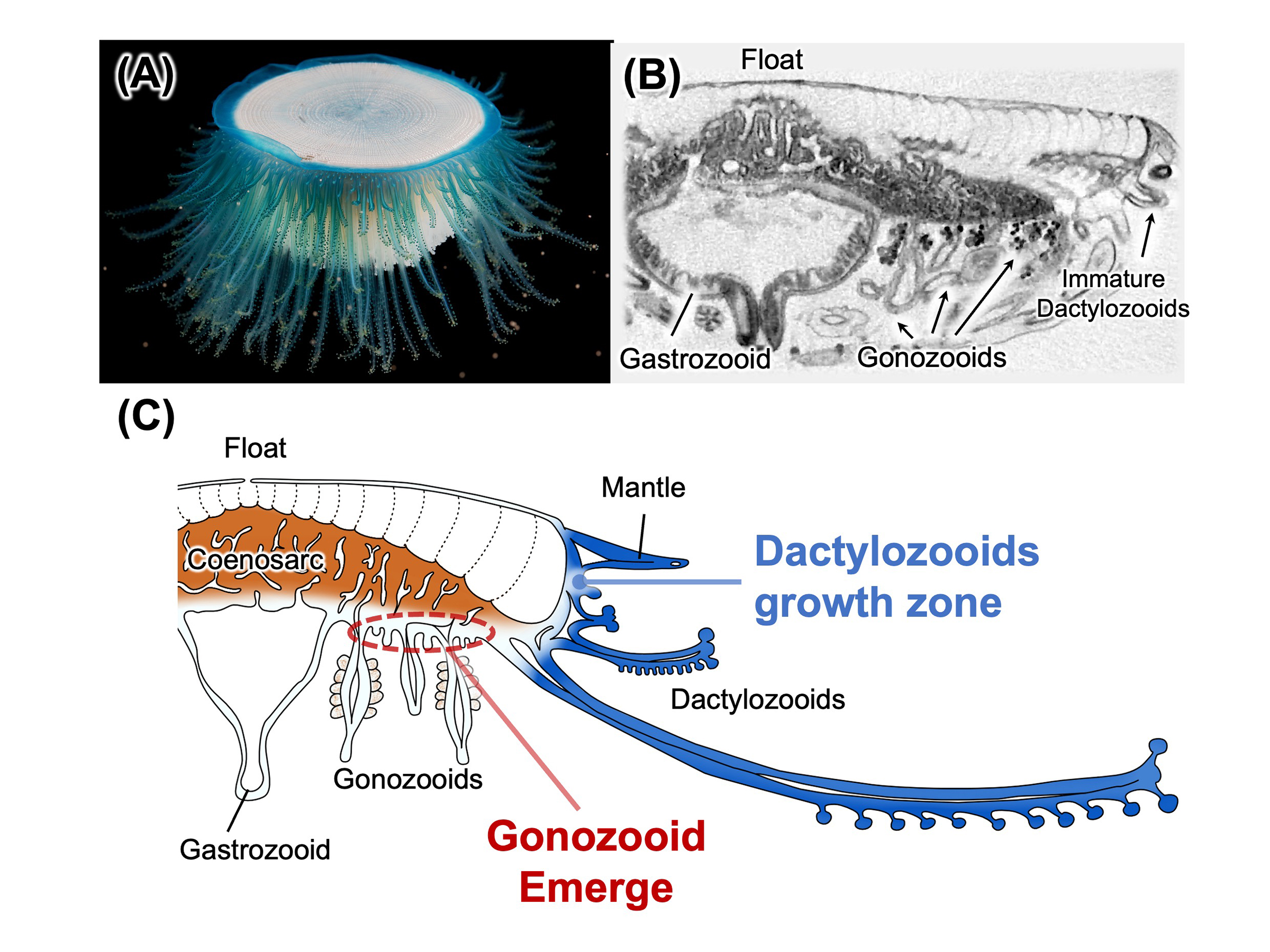
Figure: (A) Colony of Porpita porpita. (B) Cross-sectional image of the colony obtained by micro-CT. (C) Schematic diagram of the internal structure of the P. porpita colony as revealed in this study. Growth zones of dactylozooids are located along the colony margin, particularly at the base of the mantle, while those of gonozooids are widely distributed within the epithelium of the coenosarc.
Euchromatin and heterochromatin: implications for DNA accessibility and transcription
Maeshima Group / Genome Dynamics Laboratory
Euchromatin and heterochromatin: implications for DNA accessibility and transcription
Katsuhiko Minami#, Adilgazy Semeigazin#, Kako Nakazato, and Kazuhiro Maeshima*
# co-first authors; * corresponding author
Journal of Molecular Biology (2025) DOI:10.1016/j.jmb.2025.169270
In higher eukaryotic cells, such as those in humans, genomic DNA wraps around core histone proteins to form nucleosomes. These nucleosomes further fold into condensed chromatin domains that exhibit a range of folding patterns—from transcriptionally active euchromatin to inactive heterochromatin. Over the past 15 years, research, including that conducted by the authors, has revealed that these chromatin domains display liquid-like behaviors. Katsuhiko Minami (Postdoctoral Fellow, former SOKENDAI student, and JSPS Research Fellow DC2), Adilgazy Semeigazin (SOKENDAI student and MEXT Scholar), Kako Nakazato (SOKENDAI student), and Professor Kazuhiro Maeshima of the Genome Dynamics Laboratory have discussed in this paper the physical properties and dynamic behaviors of chromatin in living cells. They have further examined how these physical characteristics are linked to the regulation of gene transcription and DNA replication from the perspective of “DNA accessibility.”
Their recent findings, based on single-nucleosome imaging (paper link or NIG press release), revealed that within condensed chromatin domains, euchromatin behaves more like a liquid, while heterochromatin behaves more like a gel. These physical properties directly impact how large proteins can penetrate into the chromatin environment (accessibility), and they are considered to play critical roles in regulating genome functions such as transcription, DNA replication, and repair (see figure). The authors also discussed that euchromatin and heterochromatin have comparable accessibility on a long time scale, based on findings from DNA adenine methyltransferase (Dam) experiments. This paper is published in the Journal of Molecular Biology special issue, “Imaging of the Central Dogma.”
This work was supported by the Japan Society for the Promotion of Science (JSPS) and MEXT KAKENHI grants (JP23K17398, JP24H00061, JP23KJ0998), the Platform for Advanced Genome Science (PAGS; JP22H04925), JST SPRING (JPMJSP2104), JSPS Research Fellowship (23KJ0998), and the Takeda Science Foundation.
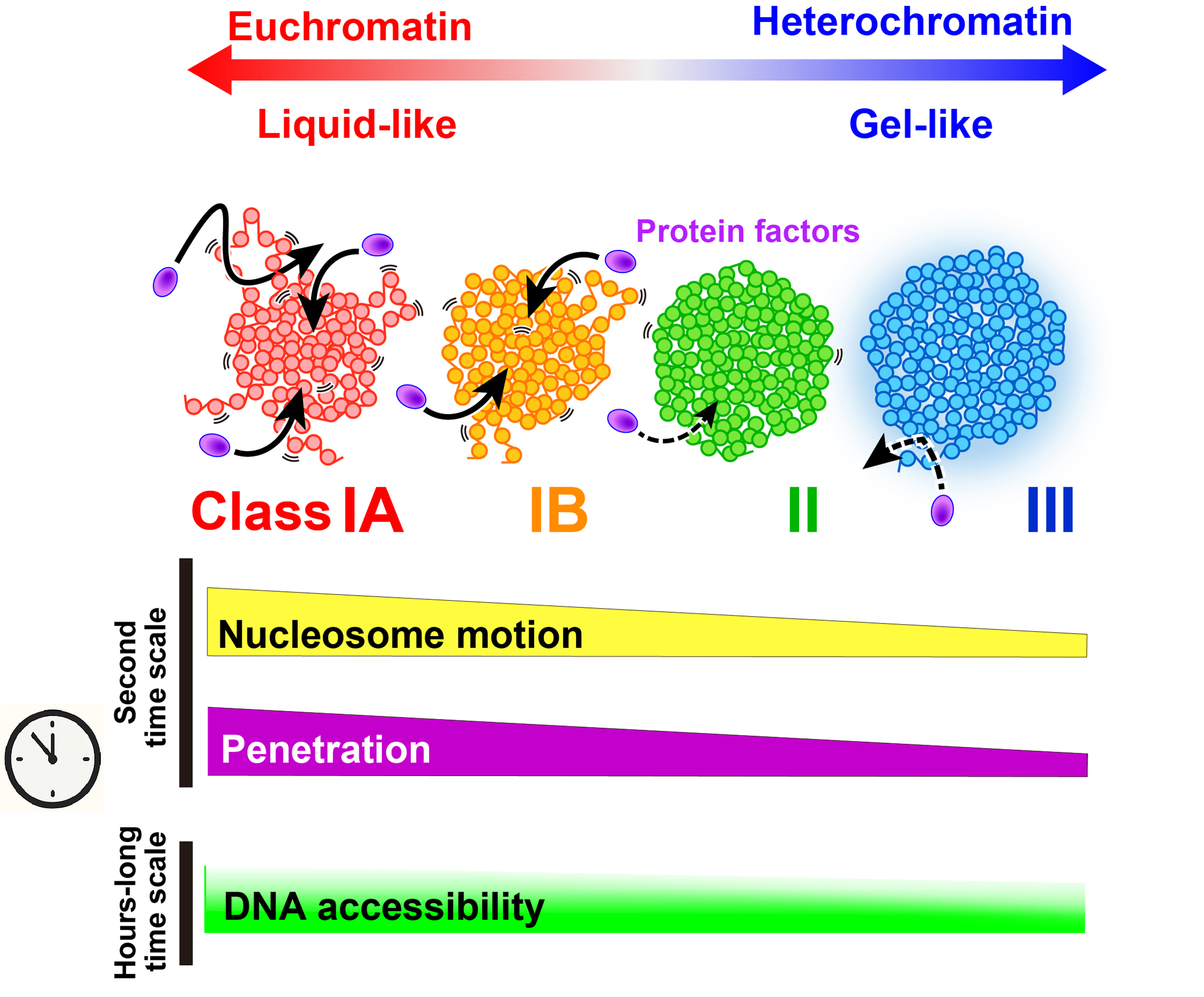
Figure: Physical properties and accessibility of euchromatin and heterochromatin
Euchromatic (actively transcribed) nucleosomes fluctuate like a liquid, while heterochromatin (transcription is repressed) behaves like a gel. Such local nucleosome fluctuations facilitate protein access to chromatin domains on a short time scale. Euchromatin and heterochromatin have comparable accessibility on a long time scale.
Efficient production of recombinant multi-subunit proteins using the Tol2 transposon
Kawakami Group / Laboratory of Molecular and Developmental Biology
Production of multi-subunit proteins in CHO cells by transposase-mediated integration of subunit-splitting vectors.
Keina Yamaguchi, Risa Ogawa, Masayoshi Tsukahara and Koichi Kawakami
Scientific reports (2025) 15, 18512 DOI:10.1038/s41598-025-03301-3
Chinese Hamster Ovary (CHO) cells are widely used for therapeutic protein production. We previously developed a method using the Tol2 transposon system to efficiently establish protein-producing cell lines via multiple genomic integrations of the gene of interest. In this study, we introduced two separate vectors—one carrying the light chain (LC) gene and the other the heavy chain (HC) gene—into CHO cells, rather than a single vector with both genes. As a result, we obtained cell lines that stably produced monoclonal antibodies for up to 12 weeks and showed high productivity in fed-batch culture. Each cell line exhibited variable copy numbers of integrated LC and HC vectors, depending on the antibody type. High-producing lines had optimal ratios of LC and HC gene copies. Notably, optimization was achieved even when the drug resistance gene was present only in the HC vector. These findings highlight the potential of the Tol2 system for efficient production of monoclonal antibodies and other multi-subunit proteins.
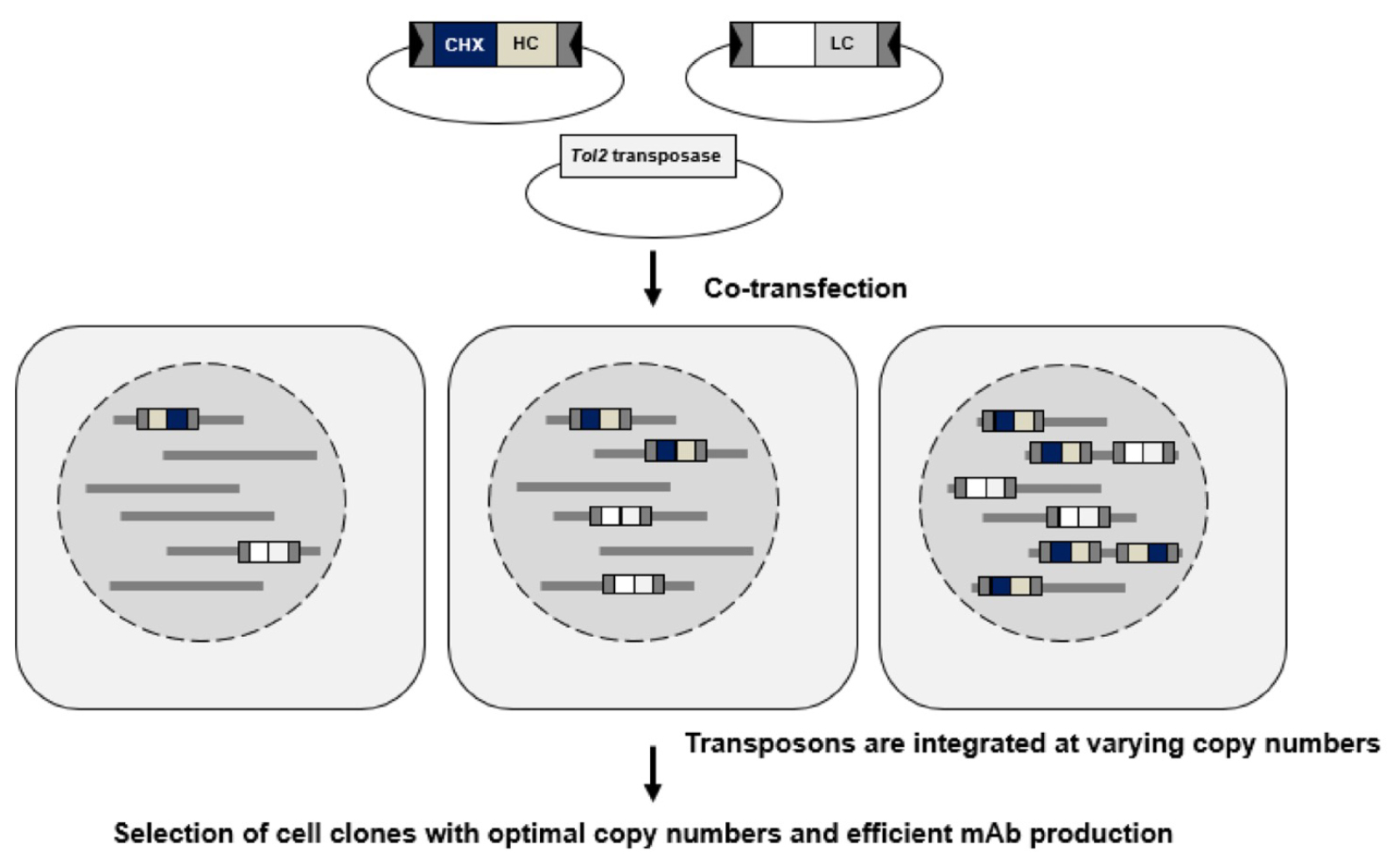
Figure: Overview of the method.
Separate transposon vectors carrying the heavy chain (HC) and light chain (LC) genes are co-transfected into CHO cells. The drug resistance gene (cycloheximide resistance) is present only in the LC vector. In the transfected cells, the HC and LC vectors are integrated into the host genome in varying copy numbers. Cell lines carrying optimal copy numbers of both HC and LC vectors can be selected as cell lines that efficiently produce monoclonal antibodies.
Algal cells strategically slack off to avoid the cost of photosynthesis.
Miyagishima Group / Symbiosis and Cell Evolution Laboratory
Costs of photosynthesis and cellular remodeling in trophic transitions of the unicellular red alga Galdieria partita
Shota Yamashita, Shunsuke Hirooka, Takayuki Fujiwara, Baifeng Zhou, Fumi Yagisawa, Kei Tamashiro, Hiroki Murakami, Koichiro Awai, and Shin-ya Miyagishima
Communications Biology (2025) 8, 891 DOI:10.1038/s42003-025-08284-5
As in plastid differentiation in land plants, some unicellular algae reversibly remodel photosynthetic plastids into a colorless heterotrophic state (bleaching) in the presence of organic carbon sources. To understand these mechanisms and their significance, we performed comparative omics analyses on the photoautotrophic and heterotrophic states and their transitions in the genetically tractable red alga Galdieria partita. Photoautotrophic cells require 1.5, 1.3 and 1.7 times more nitrogen, protein, and fatty acids than heterotrophic cells. In the photoautotrophic cells, plastid- and nucleus-encoded proteins for photosynthesis are highly synthesized, while in the heterotrophic state, cytoplasmic and mitochondrial proteins are more abundant, enabling 1.6 times faster growth. Changes in non-plastid metabolic enzymes are limited, with some upregulated in the photoautotrophic state to support fatty acid and glycolipid synthesis in the plastid for thylakoid membranes. In contrast, solute transporters show broader changes. Bleaching occurs upon adding certain sugars or sugar alcohols, regardless of light, not by active digestion of photosynthetic machinery, but by dilution due to suppressed synthesis at the transcriptional level and faster cell growth. Thus, when assimilable organic carbon is available, the cells repress the synthesis of proteins, lipids, and pigments for photosynthesis, reallocating resources to promote faster growth.
This study was supported by JSPS KAKENHI (22K15166, 24KJ0224, 24H00579), and JST-MIRAI Program (JPMJMI22E1).
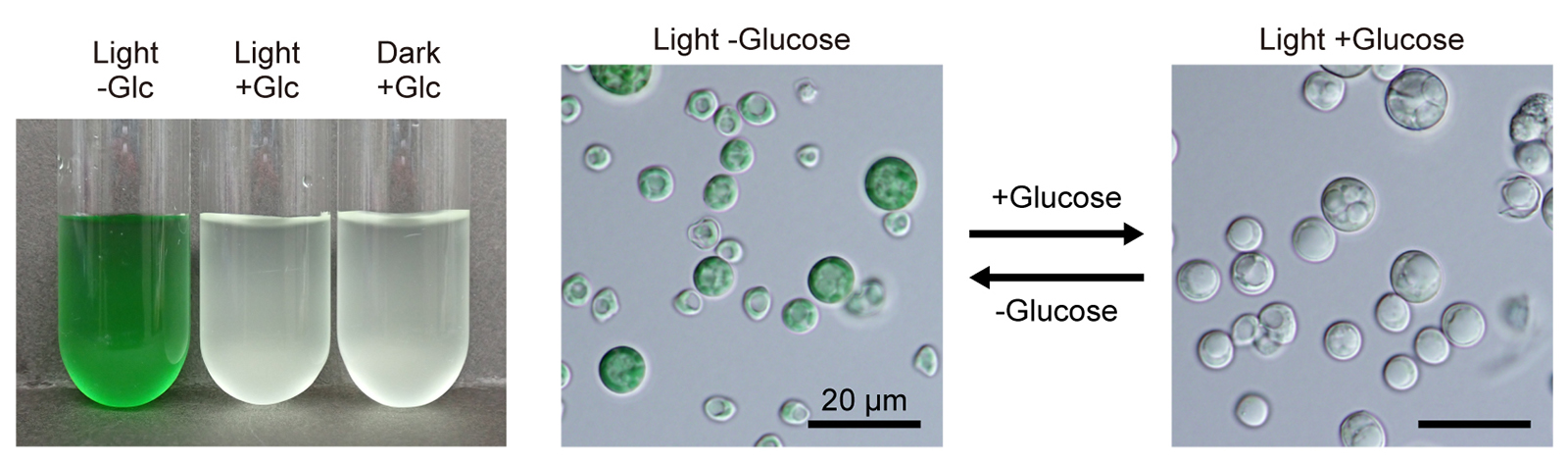
Figure: Liquid culture (left) and micrographs of cells (right) of Galdieria partita grown under light or dark conditions with or without exogenous glucose. Modified from Fig. 1A and B of the paper.















