Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Cataloging the availability of whole genome sequence information for endangered species in Japan: where is the cold spot?
Kuraku Group / Molecular Life History Laboratory
Genome assembly catalog for species in the Japanese Red List: unlocking endangered biodiversity through genomic inventory
Kryukov, K., Nakahama, N., and Kuraku, S.
F1000Research (2024) 13, 583 DOI:10.12688/f1000research.149793.2

Genomic information on another subspecies of the same species, Vespa crabro crabro, was recently obtained by a British institute and shown to be consistent with the previously reported karyotype (2n=50).
Whole genome sequence reading, which can be regarded as a catalog of DNA information, is a prerequisite for an accurate understanding of the genetic diversity that is key to the survival of a species; DNA sequences also contain information on the molecular basis that determines the ecological, morphological, and behavioral characteristics of a species. In both ways, whole genome sequencing is essential for understanding biodiversity, yet this information is available for only a handful of species. Dr. Kirill Kryukov of Joint Support-Center for Data Science Research of Research Organization of Information and Systems (ROIS-DS), Dr. Naoyuki Nakahama of the University of Hyogo and Museum of Nature and Human Activities, Hyogo, and Dr. Shigehiro Kuraku of the National Institute of Genetics have therefore focused their attention on rare species among the wild organisms living in Japan, and have developed a database, Genome sequence data availability for the Japanese Red List, which lists the accumulation status of whole genome sequence information.
This database catalogs the registration status of NCBI whole genome sequence information for rare species based on the “Red List 2020 of the Ministry of the Environment (In Japanese only)” and the “Red List of Marine Organisms of the Ministry of the Environment”, and the summarized information is made freely accessible online. The information can be viewed as a table in a web browser. The status of accumulation can be checked at a glance not only by species or subspecies, but also by taxonomic units such as “mammals,” “insects,” and “fungi” (Fig. 1). The system is also equipped with a function to regularly update the display content to reflect new information registered daily from all over the world. In addition, by downloading the files in TSV format, it can be used for secondary data analysis.

Figure1: Status of genome sequence data availability by taxon
One of the tables from the the top page of the database. The rightmost column shows the percentage of species for which genome information has been developed among the species considered to be rare in Japan. The percentage is generally very low, although it varies from taxon to taxon.
Whole genome sequencing requires not only suitable samples for extracting high molecular weight DNA and capturing the nuclear 3D structure of DNA molecules, but also the technical capability to develop sequence information that can withstand extensive use. Although numerous technological innovations and optimizations have reduced the cost and time required for calculations, the cost sometimes runs into the millions of yen, depending on the total number of bases in the genome, and also requires the labor of specialized technical personnel. Despite these limitations, it is important to steadily accumulate information, and there are many cases overseas where national governments are taking the initiative in organizing the priorities of indigenous species and preemptively acquiring genome information. The product of this study are expected to serve as a guide for Japan to vigorously promote such efforts by identifying priorities based on the recognition of the bias in the sequence data availability, and to efficiently acquire information in a more preemptive manner.
Establishment of mouse lines useful for endogenous protein degradation.
Saga Group / Mammalian Development Laboratory
Kanemaki Group / Molecular Cell Engineering Laboratory
Establishment and characterization of mouse lines useful for endogenous protein degradation via an improved auxin-inducible degron system (AID2).
Makino-Itou H, Yamatani N, Okubo A, Kiso M, Ajima R, Kanemaki MT, Saga Y.
Development, Growth and Differentiation (2024) Sep;66(7):384-393. DOI:10.1111/dgd.12942
The development of new technologies opens new avenues in the research field. Currently, conditional gene knockout strategies are employed to examine temporal and spatial gene function. However, phenotypes are sometimes not observed because of the time required for depletion due to the long half-life of the target proteins. Protein knockdown using an improved auxin-inducible degron system, AID2, overcomes such difficulties owing to rapid and efficient target depletion. Here, we established several mouse lines useful for AID2-medicated protein knockdown, which include knock-in mouse lines in the ROSA26 locus; one expresses TIR1(F74G), and the other is the reporter expressing AID-mCherry. We also established a germ-cell-specific TIR1 line and confirmed the protein knockdown specificity. In addition, we introduced an AID tag to an endogenous protein, DCP2 via the CAS9-mediated gene editing method. We confirmed that the protein was effectively eliminated by TIR1(F74G), which resulted in the similar phenotype observed in knockout mouse within 20 h.
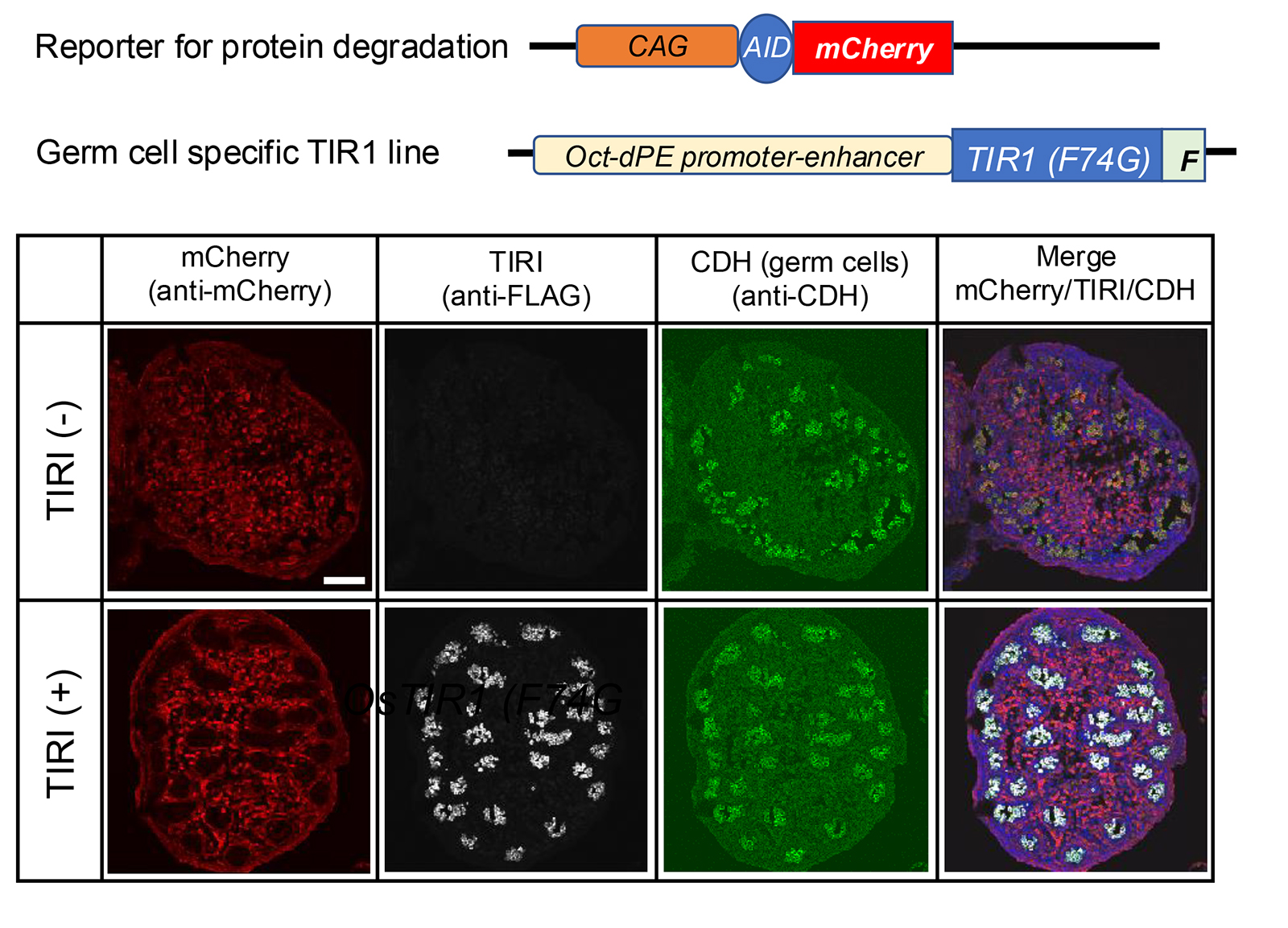
Figure: The germ-cell-specific TIR1 line was crossed with the reporter line TG-CAG-AID-mCherry. Immunofluorescence images of embryonic testes (E15.5) prepared from a pregnant CAG-AID-mCherrymother crossed with an Oct-dPE-TIR1(F74G)-FLAG male. TIR1(-) means sample without Oct-dPE-TIR1(F74G) and TIR1(+) means double transgenic sample. The mother was subjected to 5-Ph-IAA injection at E14.5. Antibodies used were anti-mCherry, anti-FLAG, and anti-E-cadherin (CDH). The scale bar indicates 100 mm.
Appointment of the New Director-General of the National Institute of Genetics
October 24, 2024
Upon the expiration of the term of the present Director-General of the National Institute of Genetics (NIG), Research Organization of Information and Systems (ROIS) has decided to appoint Dr. Shigeru Kondo as the next Director-General of the NIG, through the deliberation of the Education and Research Council.
Dr. Kondo will arrive at his post on the 1st of December, 2024.
Term of Office: From the 1st of December, 2024 to the 30th of November, 2028 (a total of 4 years)
“Dean’s Award” awarded to Genetics Program student Bhim Bahadur Biswa
Mr. Bhim Bahadur Biswa, who graduated from SOKENDAI in September 2024 as a member of the Mouse Genomics Resource Laboratory, has been awarded the School of Life Science “Dean’s Award” for the first semester of 2024.
The Dean’s Award recognizes degree recipients who have conducted research worthy of commendation and reported their accomplishments in an outstanding doctoral thesis. The award was presented on September 27, 2024 during the graduation ceremony. In addition to the Dean’s Award, Mr. Biswa has also been awarded the Genetics Program’s Morishima Award.
・Thesis title : Role of gut bacteria in domestication of mice
Mr. Biswa has provided the following statement regarding the award.
“I am deeply honored to receive the Dean’s Award from the School of Life Sciences, SOKENDAI University, for my PhD research. This recognition underscores the significance of our work in understanding the role of the gut microbiome in the animal domestication process. I am immensely grateful for the guidance and support from my supervisors and lab members, as well as the collaborative environment fostered by the National Institute of Genetics, which has been crucial in advancing this project. This award motivates me to continue pursuing innovative research with the potential to make a lasting impact in the scientific community. “

Mr. Biswa, Bhim Bahadur (right) & Associate Professor Koide
Swing the cell to know the physics inside
Press release
Live-cell imaging under centrifugation characterized the cellular force for nuclear centration in the Caenorhabditis elegans embryo.
Makoto Goda, Michael Shribak, Zenki Ikeda, Naobumi Okada, Tomomi Tani, Gohta Goshima, Rudolf Oldenbourg, Akatsuki Kimura
Proceedings of the National Academy of Sciences (PNAS) (2024) 121 (43), e2402759121 DOI:10.1073/pnas.2402759121
![]() Press release (In Japanese only)
Press release (In Japanese only)
Genomic DNA containing genetic information is stored in the cell nucleus. The nucleus is often located near the center of the cell. This means that there is a force inside the cell that moves and maintains the nucleus at the center. Measuring the amount of force is a challenging task. In this study, the researchers succeeded in measuring the force that maintains the cell nucleus in the center by applying centrifugal force to the cell using a special microscope called centrifuge polarizing microscope (CPM). CPM enables researchers to observe cells while rotating at a high speed. Researchers found that when a cell is rotated at a high speed, the cell nucleus is displaced from the center of the cell. The greater the centrifugal force, the greater the displacement of the nucleus from the center of the cell. Another special microscope, called an orientation-independent differential interference contrast (OI-DIC) microscope, revealed the mass density of the cell nucleus and thus enabled the researcher to calculate the centrifugal force acting on the nucleus. From this relationship between force and displacement, the researchers succeeded in quantifying the tiny force generated inside the cell to keep the nucleus centered. Cells are crowded with high concentrations of large molecules such as proteins. It has been a mystery how a large structure, such as the cell nucleus, can move inside crowded cells. This research unraveled part of this mystery using CPM and OI-DIC microscopes through the international collaboration.
The Hox patterning system underlying the pectoral fin formation was established before the divergence of ray-finned and lobe-finned fishes
Technical Section / Phenotype Research Center / Cell Architecture Laboratory
The functional roles of zebrafish HoxA– and HoxD-related clusters in the pectoral fin development
Mizuki Ishizaka, Akiteru Maeno, Hidemichi Nakazawa,, Renka Fujii, Sae Oikawa, Taisei Tani, Haruna Kanno, Rina Koita, and Akinori Kawamura
Scientific Reports (2024) 14, 23602 DOI:10.1038/s41598-024-74134-9
Although the pectoral fins of ray-finned fishes and the forelimbs of tetrapods differ in morphology and function, they share a homology with a common evolutionary origin. Comparing the developmental mechanisms of these structures is expected to provide insights into the evolution of vertebrate appendage formation. Analysis of knockout mice has shown that the deletion of HoxA and HoxD clusters, including Hox 9-13 genes results in significant shortening of the forelimbs. Similarly, deleting hox13 genes in HoxA and HoxD-related clusters of zebrafish also led to morphological abnormalities in the pectoral fins. However, the role of hox genes other than hox13 in pectoral fin formation remains unknown. In this study, we generated multiple zebrafish mutants with combinatorial deletions of HoxA and HoxD-related clusters and found that the pectoral fins in these mutants were significantly shortened, mirroring the abnormalities observed in the knockout mice. Furthermore, detailed analysis of the pectoral fins of surviving adult hox mutant zebrafish using micro-CT scans revealed morphological abnormalities in regions of the pectoral fins that are homologous to the forelimbs. These results further support the hypothesis that the formation of pectoral fins utilizing Hox genes in HoxA and HoxD clusters was established in common ancestors before the divergence of ray-finned and lobe-finned fishes.
This research was conducted by a group led by Associate Professor Akinori Kawamura of the Division of Life Science, Graduate School of Science and Engineering, Saitama University, with support from NIG-JOINT (38A2019, 7A2020, 66A2021, 18A2022, 31A2023).
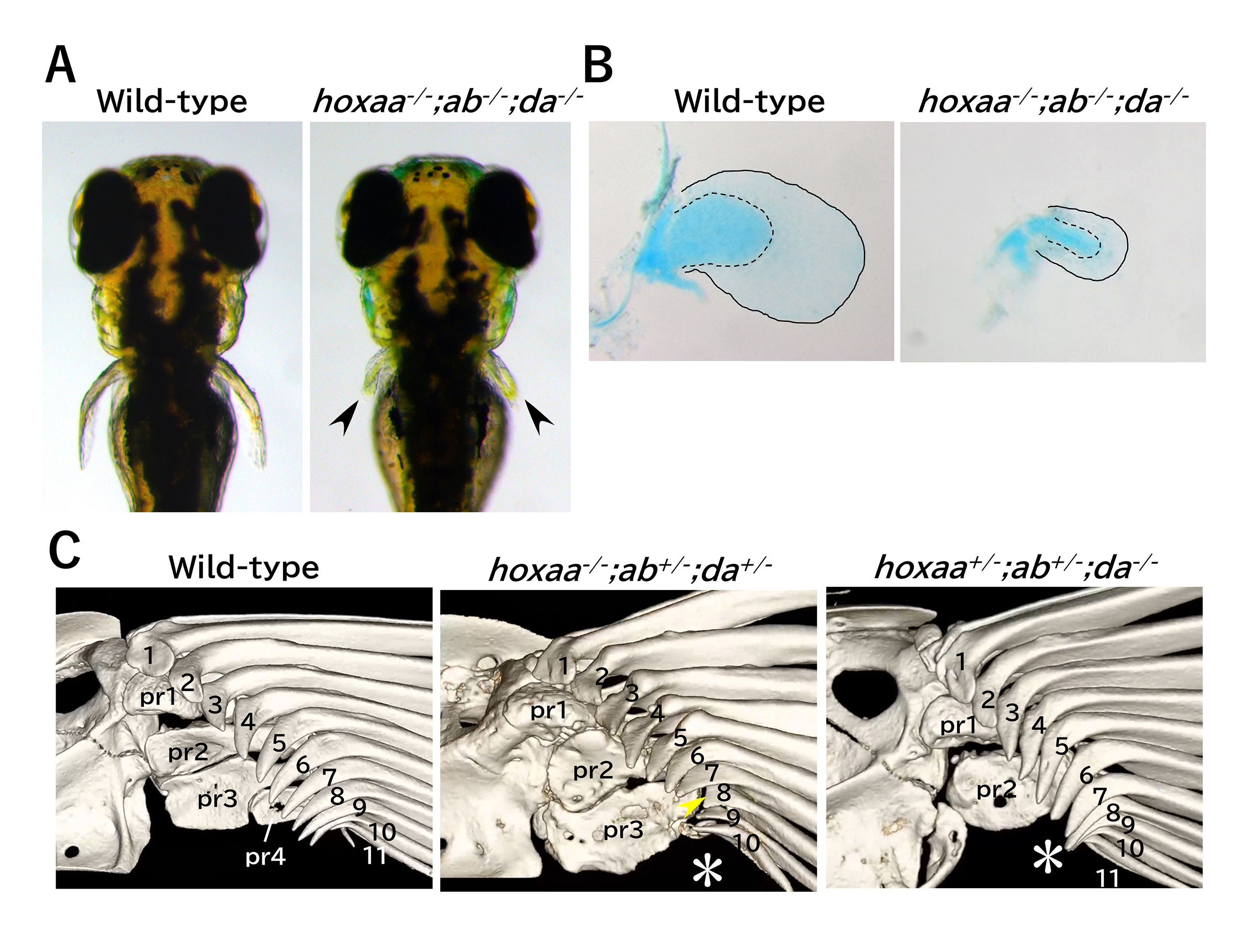
Figure: (A) Significant shortening of the pectoral fin in zebrafish hoxaa;ab;da cluster-delted mutants. 3-day post-fertilization. (B) Stained chondrocytes of the dissected pectoral fin (5-day). (C) Abnormal pectoral fin morphology in hox mutant adults revealed by micro-CT scan analysis.
Guideline for Application for 2025 NIG-JOINT(Joint Researchi and Research Meeting) *Application was closed
Guideline for Additional Application for
2024 NIG-JOINT(Joint Researchi-(A))
(Application deadline: noon(12:00pm)
on Friday, May 31st, 2024)
Guideline for Additional Application for
2024 NIG-JOINT(Joint Researchi-(A))
(Application deadline: noon(12:00pm)
on Friday, May 31st, 2024)
Guideline for Additional Application for
2024 NIG-JOINT(Joint Researchi-(A))
(Application deadline: noon(12:00pm)
on Friday, May 31st, 2024)















