Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
NIG-GS (NIG Global Scholar) selection
Genome editing is now possible in wild mouse strains!
Koide Group / Mouse Genomics Resource Laboratory
Efficient genome editing in wild strains of mice using the i-GONAD method
Yuji Imai, Akira Tanave, Makoto Matsuyama, and Tsuyoshi Koide
Scientific Reports (2022) 12, 13821 DOI:10.1038/s41598-022-17776-x
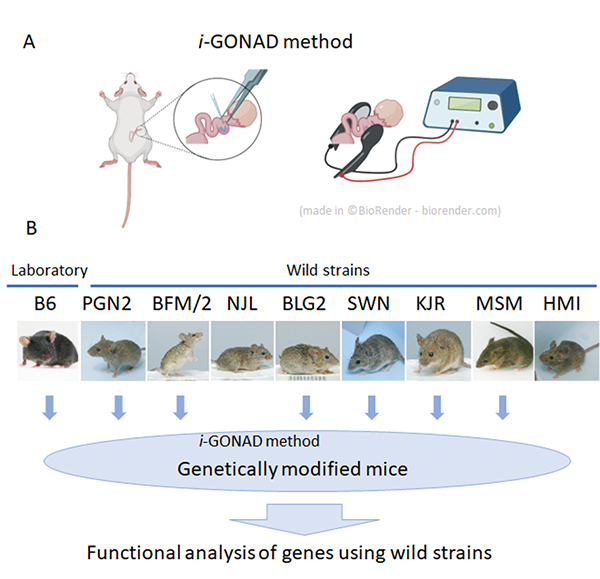
At the National Institute of Genetics, nine wild mouse strains have been established. These strains have characteristics not found in laboratory strains, such as marked genetic differences between different strains and behavior characteristic of wild mice.
These series of wild strains, named the Mishima Battery, are provided to researchers in Japan and overseas as highly unique resources, and are used for various research in the fields of cancer, immunology, development, and behavior. However, in spite of these excellent characteristics, wild strains have the problem of being difficult to apply genetic engineering technology.
In collaboration with Shigei Medical Research Institute and RIKEN, technical staff Yuji Imai and Associate Professor Tsuyoshi Koide of the Mouse Genomics Resource Laboratory have applied genome-editing using i-GONAD method, which does not involve any ex vivo manipulation of unfertilized or fertilized egg. The group showed that it is possible to efficiently modify genes in most wild strains by using this method.
First, in vitro fertilization was performed on the experimental strain C57BL/6 (B6 strain) and nine wild strains to investigate the efficiency of in vitro fertilization necessary for general genome editing experiments. As a result, it was found that only two wild strains were able to perform in vitro fertilization with the similar efficiencies as the B6 strain, and the other strains were extremely inefficient. Therefore, we applied a method called i-GONAD, developed by Professor Masato Otsuka of Tokai University, to wild strains, which performs genome editing without ex vivo manipulation of fertilized eggs. As a result, we succeeded in performing genome editing in 7 out of the 9 strains examined. This result indicates that it has become possible to efficiently perform genetic modification using wild strains in the future, and the use of wild strains in many research fields can be expected.
Sequential accumulation of dynein and its related proteins during mitosis and a possible sequential activation mechanism
Kimura Group / Cell Architecture Laboratory
Sequential accumulation of dynein and its regulatory proteins at the spindle region in the Caenorhabditis elegans embryo
Takayuki Torisawa, & Akatsuki Kimura
Scientific Reports (2022) 12, 11740 DOI:10.1038/s41598-022-15042-8
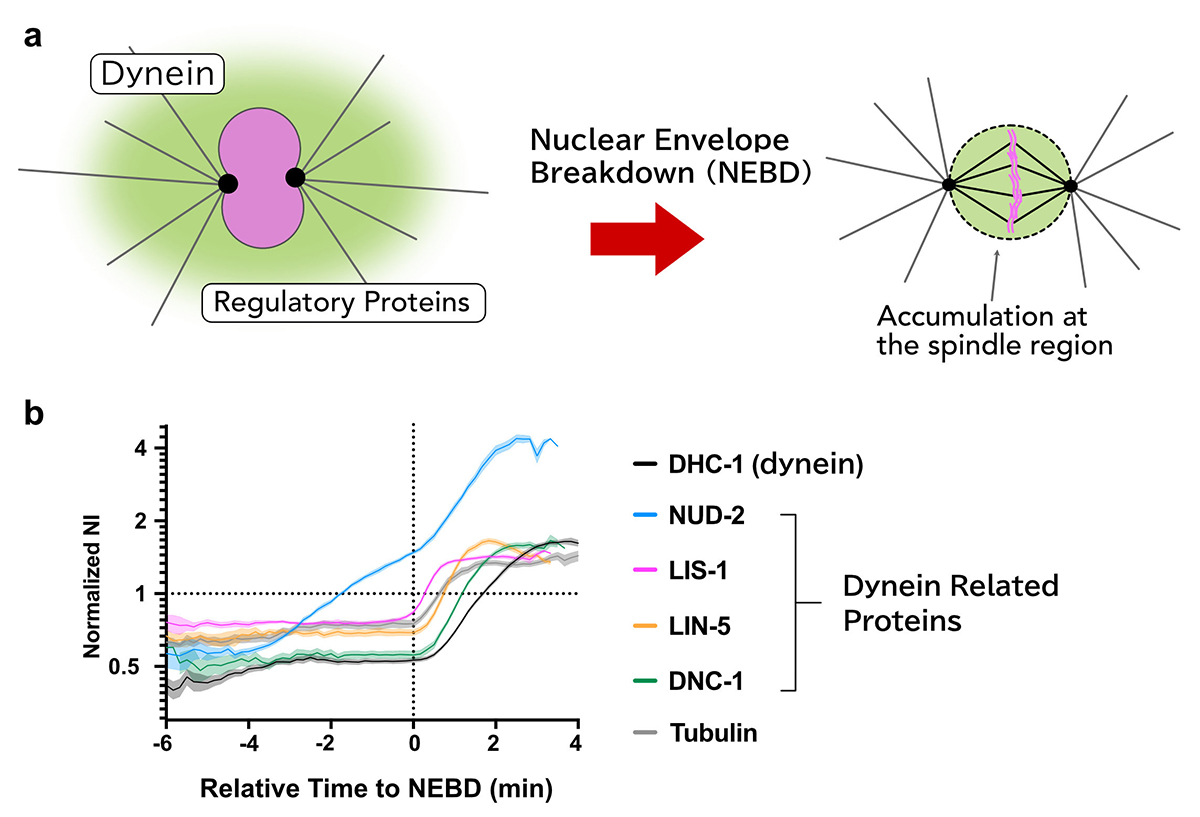
Cytoplasmic dynein is a molecular motor responsible for various cellular activities, including intracellular transport and cell division. To achieve various functions, dynein needs to be regulated by other proteins, and it has still been elusive how these regulations are achieved in many cellular contexts.
Using the early embryos of Caenorhabditis elegans, we focused on the spatiotemporal regulation of dynein during mitosis, where dynein and its regulatory proteins translocated from the cytoplasm to the spindle region, and observed the dynamics of dynein and the regulatory proteins.
We revealed that there are i) selectivity, ii) varieties in the accumulation sites, and iii) the order of accumulation in the accumulation dynamics in the spindle region.
Furthermore, we found that the accumulation of NUD-2 was unique among the dynein regulators we analyzed. NUD-2 started to accumulate before NEBD (pre-NEBD accumulation). Using a protein injection approach, we revealed that the C-terminal helix of NUD-2 was responsible for pre-NEBD accumulation. These findings suggest a fine temporal control of the subcellular localization of regulatory proteins.
This work was supported by JSPS Grants-in-Aid for Scientific Research JP19K16094, JP18H02414, JP18H05529, and JP18KK0202.
A phase transition for chromosome transmission when cells divide
Maeshima Group / Genome Dynamics Laboratory
A phase transition for chromosome transmission when cells divide
Kazuhiro Maeshima
Nature 2022 August 03 DOI:10.1038/d41586-022-01925-3
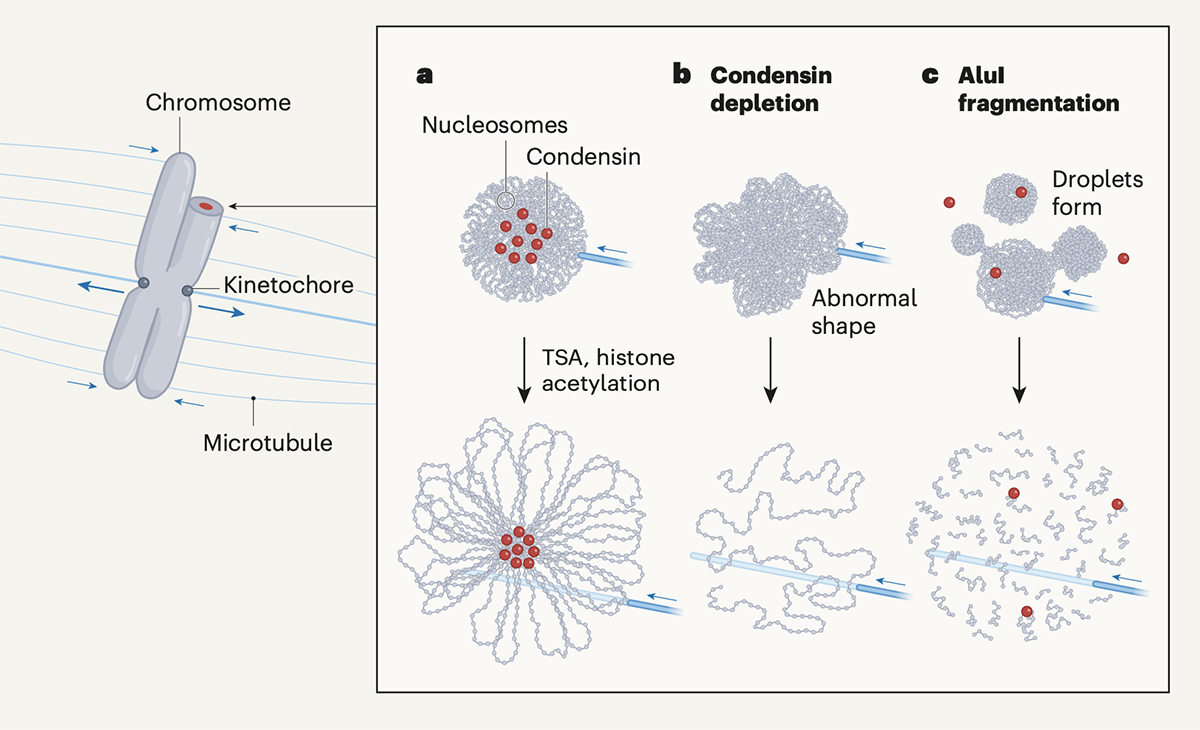
Mitotic chromosomes are the structure where DNA is tightly compacted, and they carry genetic information to be passed on to the next generation. These chromosomes are transmitted from the mother cell onto the daughter cell with physical force from microtubes and other factors. In other words, chromosomes should have mechanical resistance to endure such forces.
Recently, Daniel W. Gerlich and his colleagues have shown that chromosomes condense and gain such mechanical resistance by phase transition(Schneider et al. “A chromatin phase transition protects mitotic chromosomes against microtubule perforation” Nature 2022 doi: 10.1038/ s41586-022-05027-y). In this paper, Schneider et al. have shown that global histone deacetylation cause phase transition in mitotic chromosomes, which makes them condensed and resistant to mechanical forces. The authors have also shown that a protein complex called condensin, which was previously thought to be essential for chromosome condensation, is not involved in the condensation process itself.
Professor Kazuhiro Maeshima at Genome Dynamics Laboratory wrote a commentary on this paper in the News & Views section of Nature. Maeshima discussed how global histone deacetylation causes chromosome condensation and the role of condensin in shaping rod-like chromosomes (chromatin loop formation mechanism).