Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Canceled: The NIG International Symposium on Molecular Mechanism of Chromosome Replication
Repulsive signal contributes to proper termination of neuronal migration
Semaphorin 6A–Plexin A2/A4 interactions with radial glia regulate migration termination of superficial layer cortical neurons.
Yumiko Hatanaka, Takahiro Kawasaki, Takaya Abe, Go Shioi, Takao Kohno, Mitsuharu Hattori, Akira Sakakibara, Yasuo Kawaguchi & Tatsumi Hirata
iScience 21, pp 359-374, 2019 DOI:10.1016/j.isci.2019.10.034
Precise regulation of neuronal migration termination is crucial for the establishment of brain cytoarchitectures. However, little is known how neurons terminate migration. We found that superficial layer cortical neurons (SLNs) migrate beyond their final destination and ectopically invade layer 1 in Plexin (Plxn) A2/A4 double-knockout mice as well as Semaphorin (Sema) 6A knockout mice. Cell-targeted gene expression and conditional knockouts indicated that Sema6A on radial glial cells and PlxnA2/A4 on SLNs are involved in this process. Given that Sema6A–PlxnA2/A4 trans-interactions generally induce a repulsive reaction, our results suggest that Sema6A–PlxnA2/A4 interaction elicits repulsion and weakens migrating neuron–substrate interactions, leading to migration termination.
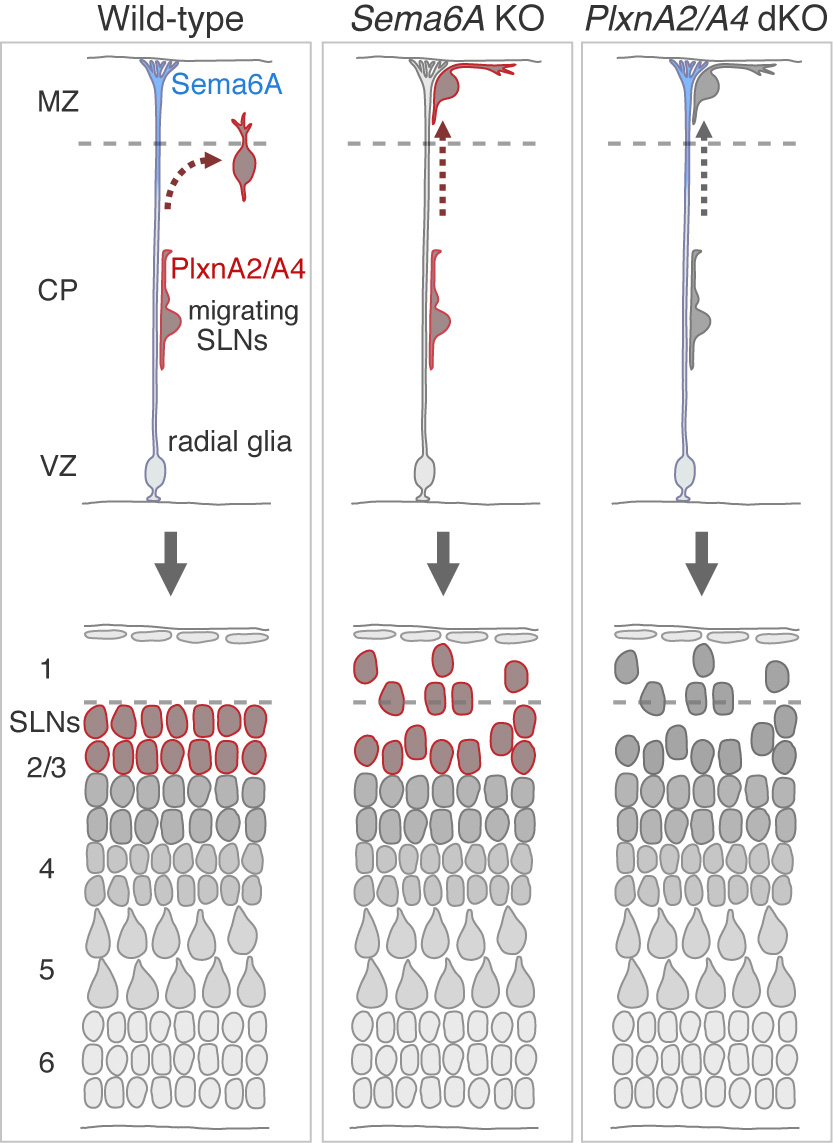
Figure: Cortical neurons migrate toward the pial surface along radial glial cell fibers. Sema6A on radial glial cells and PlxnA2/A4 on migrating neurons are necessary for proper migration termination of superficial layer cortical neurons.
A novel katanin-tethering machinery accelerates cytokinesis
Press release
A novel katanin-tethering machinery accelerates cytokinesis
Takema Sasaki, Motosuke Tsutsumi, Kohei Otomo, Takashi Murata, Noriyoshi Yagi, Masayoshi Nakamura, Tomomi Nemoto, Mitsuyasu Hasebe, Yoshihisa Oda
Current Biology 29, 1-11 DOI:10.1016/j.cub.2019.09.049
Press release (In Japanese only)
Cytokinesis is fundamental for cell proliferation. In plants, a bipolar short-microtubule array forms the phragmoplast, which mediates vesicle transport to the midzone and guides the formation of cell walls that separate the mother cell into two daughter cells. The phragmoplast centrifugally expands toward the cell cortex to guide cell-plate formation at the cortical division site. Several proteins in the phragmoplast midzone facilitate the anti-parallel bundling of microtubules and vesicle accumulation. However, the mechanisms by which short microtubules are maintained during phragmoplast development, in particular, the behavior of microtubules at the distal zone of phragmoplasts, are poorly understood. Here, we show that a plant-specific protein, CORTICAL MICROTUBULE DISORDERING 4 (CORD4), tethers the conserved microtubule-severing protein katanin to facilitate formation of the short-microtubule array in phragmoplasts. CORD4 was specifically expressed during mitosis and localized to preprophase bands and phragmoplast microtubules. Custom-made two-photon spinning disk confocal microscopy revealed that CORD4 rapidly localized to microtubules in the distal phragmoplast zone during phragmoplast assembly at late anaphase and persisted throughout phragmoplast expansion. Loss of CORD4 caused abnormally long and oblique phragmoplast microtubules and slow expansion of phragmoplasts. The p60 katanin subunit, KTN1, localized to the distal phragmoplast zone in a CORD4-dependent manner. These results suggest that CORD4 tethers KTN1 at phragmoplasts to modulate microtubule length, thereby accelerating phragmoplast growth. This reveals the presence of a distinct machinery to accelerate cytokinesis by regulating the action of katanin.
Source: Sasaki et al., (2019) Current Biology 29, 1-11, DOI: 10.1016/j.cub.2019.09.049
Summer Internship at NIG “NIGINTERN2020”
Microfocus X-ray CT (microCT) Imaging of Actinia equina (Cnidaria), Harmothoe sp. (Annelida), and Xenoturbella japonica (Xenacoelomorpha).
Microfocus X-ray CT (microCT) Imaging of Actinia equina (Cnidaria), Harmothoe sp. (Annelida), and Xenoturbella japonica (Xenacoelomorpha).
Maeno A, Kohtsuka H, Takatani K, Nakano H.
Journal of Visualized Experiments 150 e59161 1-9 DOI:10.3791/59161
Traditionally, biologists have had to rely on destructive methods such as sectioning in order to investigate the internal structures of opaque organisms. Non-destructive microfocus X-ray computed tomography (microCT) imaging has become a powerful and emerging protocol in biology, due to technological advancements in sample staining methods and innovations in microCT hardware, processing computers, and data analysis software. However, this protocol is not commonly used, as it is in the medical and industrial fields. One of the reasons for this limited use is the lack of a simple and comprehensible manual that covers all of the necessary steps: sample collection, fixation, staining, mounting, scanning, and data analyses. Another reason is the vast diversity of metazoans, particularly marine invertebrates. Because of marine invertebrates’ diverse sizes, morphologies, and physiologies, it is crucial to adjust experimental conditions and hardware configurations at each step, depending on the sample. Here, microCT imaging methods are explained in detail using three phylogenetically diverse marine invertebrates: Actinia equina (Anthozoa, Cnidaria), Harmothoe sp. (Polychaeta, Annelida), and Xenoturbella japonica (Xenoturbellida, Xenacoelomorpha). Suggestions on performing microCT imaging on various animals are also provided.
Source: Maeno A. et al, (2019) J Vis Exp. 6 Augst, DOI:10.3791/59161.
Inquiries : Technical Specialist, MAENO, Akiteru(amaeno@nig.ac.jp)
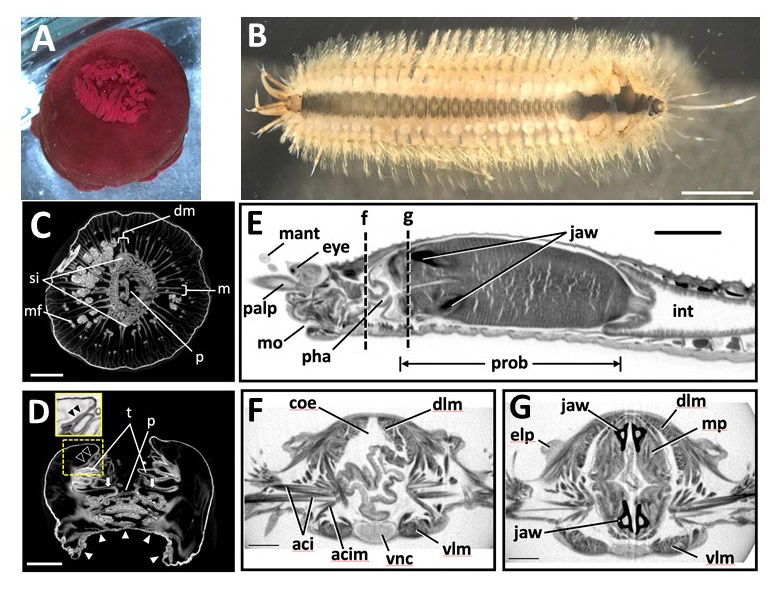
Figure: Marine invertebrate animals observed in this study. (A,B)
(A) Live anesthetized Actinia equina (Anthozoa, Cnidaria).
(B) Live anesthetized Harmothoe sp. (Polychaeta, Annelida).Most of the elytra were already missing at this stage, with only four remaining near the posterior end. Scale bars = 3 mm.
Scanned and reconstructed images of marine invertebrates. (C-G)
(C) Transverse and (D) longitudinal sections of Actinia equina. Scale bars in C, D = 3 mm.
(E-G) Harmothoe sp. (Polychaeta, Annelida). (E) Sagittal section of the anterior part. (F, G) Transverse section at the dotted lines f and g in (E). Scale bars: E = 1 mm; F, G = 0.3 mm.
Volume rendering image and transverse section movie of the whole body of Harmothoe sp. 6 sec to 16 sec: 3D volume rendering image. Top: left view, bottom: frontal view. 17 sec to 1min 42 sec: Transverse section movie. The position of the section is shown with a moving green line on the sagittal section image at top. From 17 sec to 53 sec, transverse section movie of the anterior part of the specimen generated from Pinpoint Scan is shown at the left bottom. From 1 min 7 sec to 1 min 14 sec, a 3D model made using Imaris software showing the positions of major organs is present at right top. Anatomical supervision: Masaatsu Tanaka (Kagoshima University)
This technique is one of the bases of following researches.
・ Evolution of Shh endoderm enhancers during morphological transition from ventral lungs to dorsal gas bladder
・ Regulation of internode patterning and vein anastomosis in maize stems.
・ Combination of multiple Shh enhancers controls tooth development in mouse
・Development of organs in living whole embryo/larval grafts in zebrafish
・ Enhancer adoption changes limb morphology
・When do male and female differences appear in the development of beetle horns? (National Institute for Basic Biology )
・A mouse model of human chromosomal disorder, partial trisomy distal 4q
A novel central olfactory circuit revealed by a newly developed neuronal birthdate tagging method
A Novel Birthdate-Labeling Method Reveals Segregated Parallel Projections of Mitral and External Tufted Cells in the Main Olfactory System
Tatsumi Hirata, Go Shioi, Takaya Abe, Hiroshi Kiyonari, Shigeki Kato, Kazuto Kobayashi, Kensaku Mori and Takahiko Kawasaki
eNeuro 31, ENEURO.0234-19.2019, 2019 DOI:10.1523/ENEURO.0234-19.2019
Odorant receptors form an ordered odorant map on the main olfactory bulb. This spatial representation disappears in most subsequent targets by “diffuse divergence and random convergence” of olfactory bulb projections. We revisited these projections using a newly developed method that can genetically dissect distinct subsets of olfactory bulb projection neurons based on their neuronal birthdates. Our birthdate tag analysis exposed parallel segregated projections formed by early-born mitral and late-born external tufted cells in otherwise apparently random olfactory networks. The results suggest that these parallel pathways extract unique features of information from the common olfactory input and process these features in a way similar to “color”, “orientation” or “direction” in the visual system. Importantly, the birthdate tag method can pave the way for deciphering the functional meaning of these individual pathways in the future.
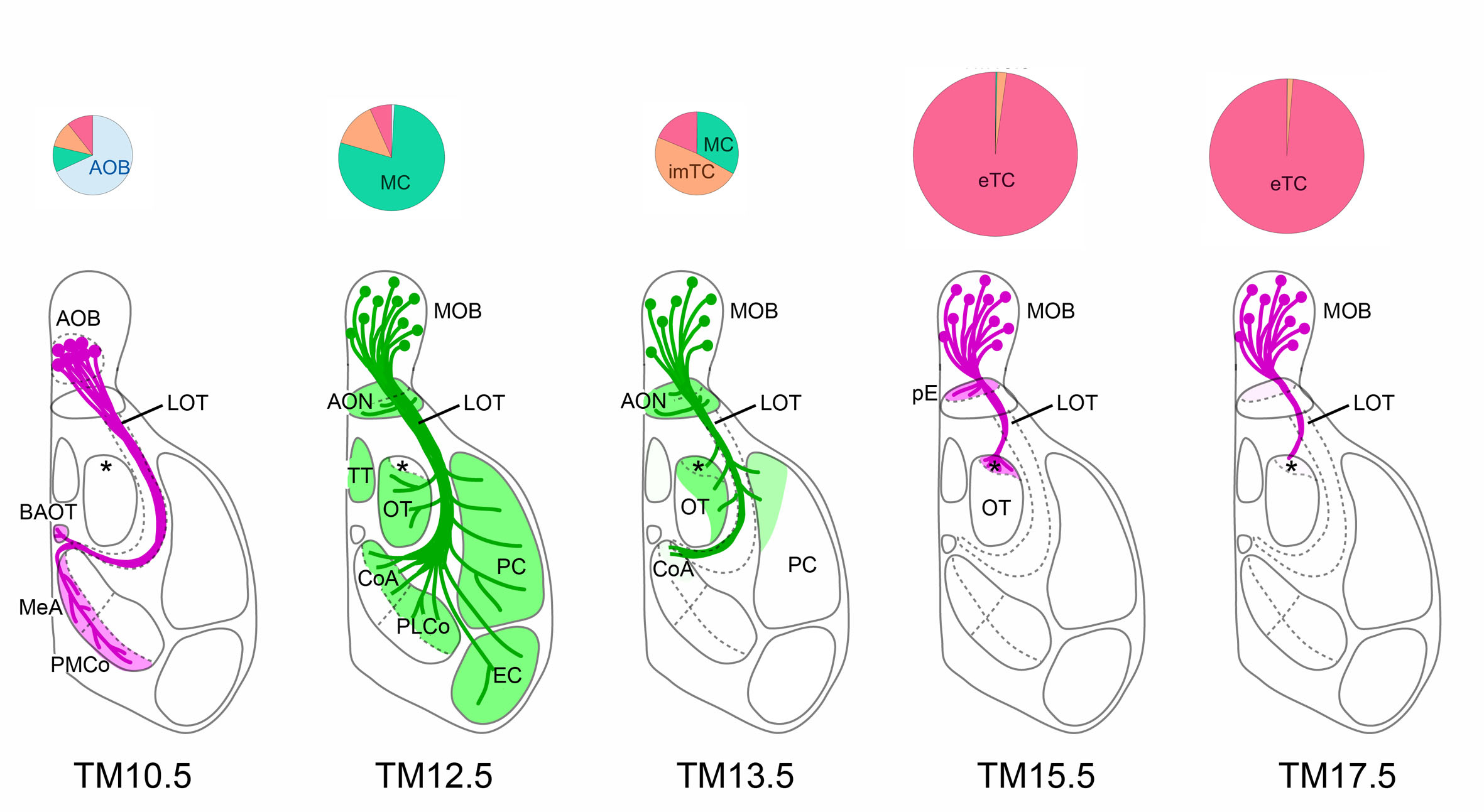
Figure: Dissection of olfactory bulb projection neurons and their axon trajectories using neuronal birthdate tagging
Depending on tamoxifen injection stages (TM10.5~17.5), different classes of neurons such as accessory olfactory bulb neurons (AOB), mitral cells (MC) or tufted cells (TC) are tagged (pie charts), and their axon trajectories are revealed (bottom diagrams).
- This method is one of the basises of“NeuroGT Database”, a brain atlas ofneurogenic tagging CreER drivers”(NeuroGT).















