Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Polyploid genomes in bacteria: regulation of replication and the biological significance
Coordination of polyploid chromosome replication with cell size and growth in a cyanobacterium.
Ryudo Ohbayashi, Ai Nakamachi, Tetsuhiro Hatakeyama, Yu Kanesaki, Satoru Watanabe, Taku Chibazakura, Hirofumi Yoshikawa, and Shin-ya Miyagishima
mBio 10(2), e00510-19 DOI:10.1128/mBio.00510-19
Homologous chromosome number (ploidy) has diversified among bacteria, archaea, and eukaryotes over evolution. In bacteria, model organisms such as Escherichia coli possess a single chromosome. In contrast, other bacteria, including cyanobacteria, maintain multiple copies of individual chromosomes (polyploid). Although a correlation between ploidy level and cell size has been observed in bacteria and eukaryotes, it is poorly understood how replication of multi-copy chromosomes is regulated and how ploidy level is adjusted to cell size. Here we show that only one or a few multi-copy chromosomes are replicated at once in the cyanobacteria Synechococcus elongatus and that this restriction depends on regulation of DnaA activity. When cell growth rate was increased or decreased, DnaA level, DnaA activity, and the number of replicating chromosomes also increased or decreased in parallel, resulting in nearly constant chromosome copy number per unit cell volume. Thus, it is suggested that the stepwise replication of the genome enables cyanobacteria to maintain nearly constant gene copy number per unit cell volume.
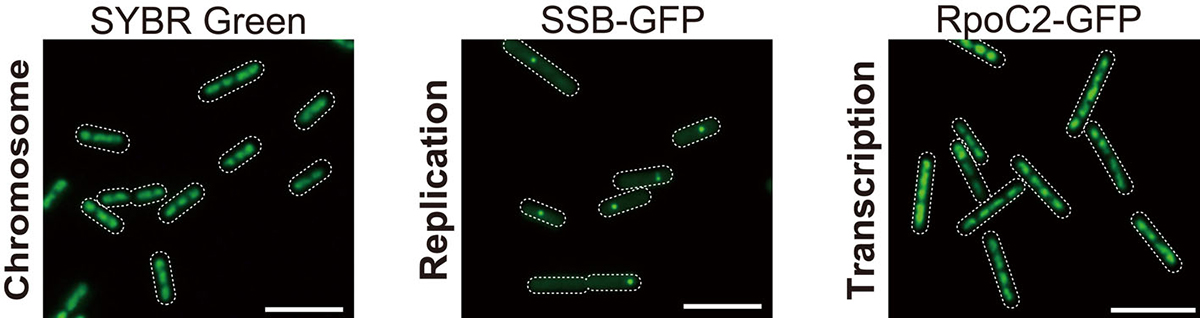
Figure. 1: Microscopic images of SYBR Green-stained nucleoids, SSB-GFP (replicating chromosomes), and RpoC2-GFP (transcribed chromosomes). All copies of chromosomes are transcribed while chromosomes are replicated one by one.
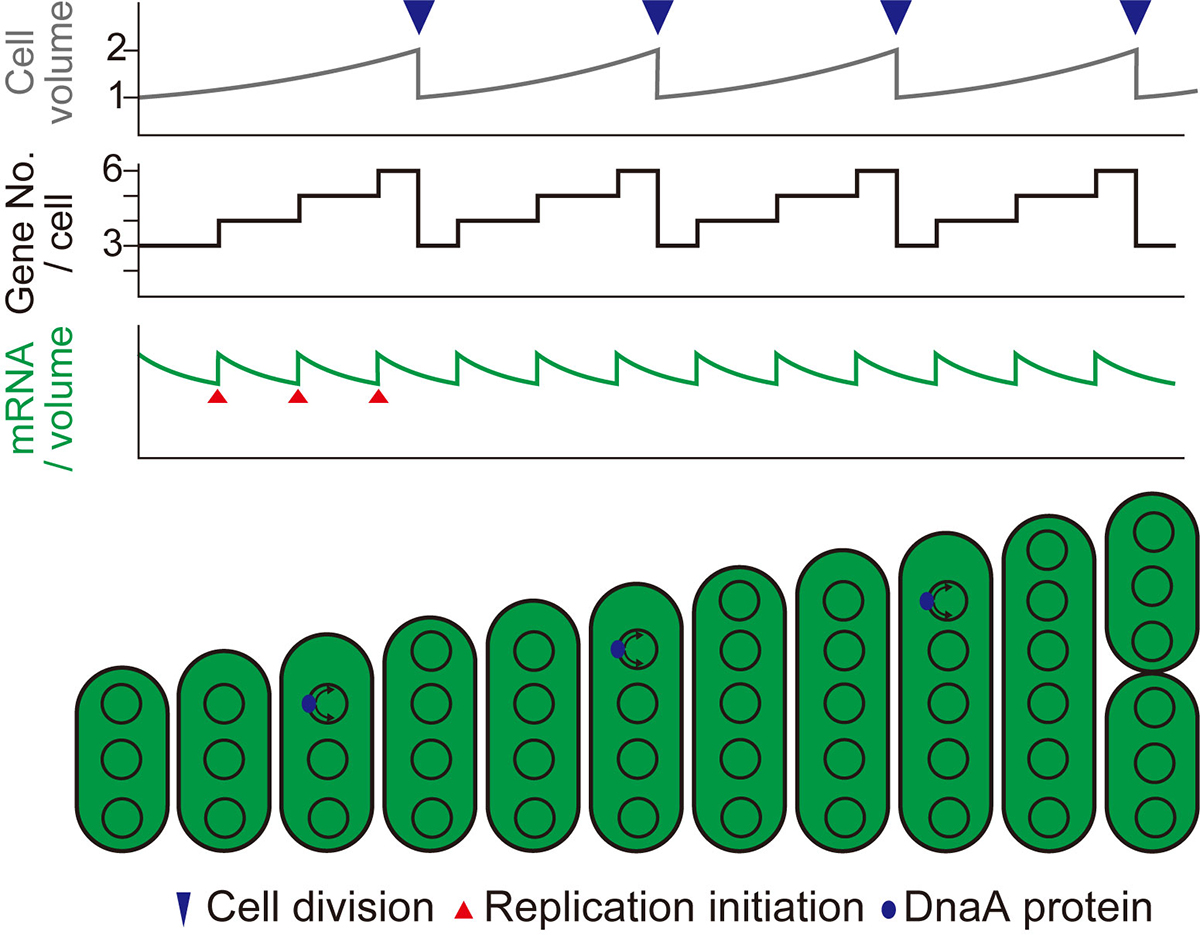
Figure. 2: Schematic diagrams showing changes in cell volume (upper), gene copy number per cell (middle), and mRNA level per unit volume (bottom) during cell growth in the case of stepwise (as observed in this study) replication of multi-copy chromosomes.
Identification of a key gene for freshwater colonization in fishes
Press release
A key metabolic gene for recurrent freshwater colonization and radiation in fishes.
Asano Ishikawa, Naoki Kabeya, Koki Ikeya, Ryo Kakioka, Jennifer N. Cech, Naoki Osada, Miguel C. Leal, Jun Inoue, Manabu Kume, Atsushi Toyoda, Ayumi Tezuka, Atsushi J. Nagano, Yo Y. Yamasaki, Yuto Suzuki, Tomoyuki Kokita, Hiroshi Takahashi, Kay Lucek, David Marques, Yusuke Takehana, Kiyoshi Naruse, Seiichi Mori, Oscar Monroig, Nemiah Ladd, Carsten J. Schubert, Blake Matthews, Catherine L. Peichel, Ole Seehausen, Goro Yoshizaki, and Jun Kitano.
Science 31 May 2019: Vol. 364, Issue 6443, pp. 886-889 DOI:10.1126/science.aau5656
EurekAlert! link about this artcle
Fishes are present in not only marine but also freshwater environments including rivers, streams, lakes, and ponds because ancestral marine fishes have colonized freshwater multiple times and diversified in the freshwater environment during evolution. However, only few fish lineages could colonize freshwater habitats. What enable some lineages to colonize freshwater?
Dr. Asano Ishikawa and Dr. Jun Kitano of Ecological Genetics Laboratory at the National Institute of Genetics, Japan, collaborated with researchers from Japan, Switzerland, USA, and Spain and asked this question using stickleback fishes (Fig. 1) as a model. They identified a key gene important for freshwater colonization, Fatty acid desaturase 2 (Fads2), encoding an enzyme involved in the synthesis of docosahexaenoic acid (DHA), a polyunsaturated fatty acid. DHA is a component of the cell membrane and is essential for growth and survival of fish. In aquatic ecosystems, marine-derived diets are rich in DHA, while diets in freshwater environments comprise limited DHA. They found that sticklebacks that successfully colonized freshwater have higher copy numbers of Fads2 and higher abilities to synthesize DHA than those that failed to colonize freshwater (Fig. 2). Analysis of publicly available whole-genome sequences of 48 fish species revealed higher Fads2 copy numbers in freshwater fish than in marine fish across diverse fish taxa.
This research was supported by JSPS KAKENHI (15H02418, 23113007, 16H06279, 26870824, 16K07469), JSPS PD (11J04816, 16J06812), Asahi Glass Foundation, Sumitomo Foundation, SNF grant (31003A 175614), and SNSF (31003A_163338, PDAMP3_123135).
This was published in Science at 2:00 p.m. U.S. Eastern Time on Thursday, 30 May 2019.

Fig: 1.A photo of a Japan Sea stickleback (Gasterosteus nipponicus) male. Photo by Yasuyuki Hata.
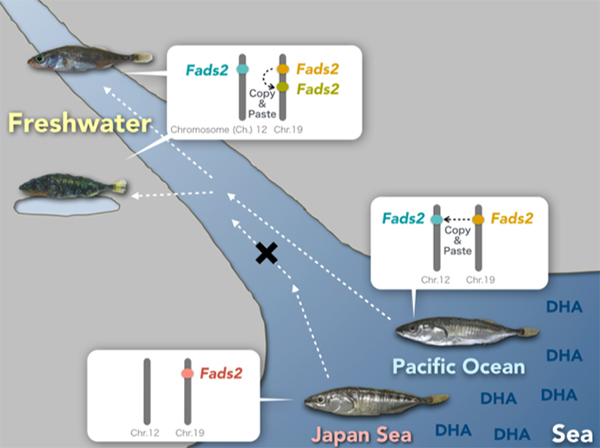
Fig: 2.Freshwater colonization and increase in Fads2 gene Japan Sea sticklebacks that could not colonize freshwater have only one Fads2 copy on Chromosome 19, while three-spined sticklebacks that could colonize have additional copy of Fads2 on Chromosome 12. Some freshwater populations of three-spined sticklebacks further duplicated Fads2 genes on Chromosome 19.
Robustness and flexibility of the chromosome segregation apparatus
Mechanically distinct microtubule arrays determine the length and force response of the meiotic spindle
Jun Takagi, Ryota Sakamoto, Gen Shiratsuchi, Yusuke T. Maeda, Yuta Shimamoto
Developmental Cell, Vol 49, pp 267-278, 2019 DOI:10.1016/j.devcel.2019.03.014
During cell division, our genome must be faithfully transmitted from the mother cell to daughter cells. This task is done by the spindle, the micron-sized bipolar force-generating machinery. Using a microfabricated force sensor and high-resolution microscopy, a team of Yuta Shimamoto at National Institute of Genetics and Yusuke Maeda at Kyushu University probed the local mechanical architecture of the spindle (Fig. 1A) and found its substantial heterogeneity; the equator and pole regions are stiff whereas its middle is more flexible and deformable (Fig. 1B). The spindle’s mechanical heterogeneity explains how this micron-sized structure can maintain its local functional architectures, such as the pole and the equator, required to pull chromosomes and determine the cell division axis, while adapting the overall structure to perturbations. Their physical approach will help understand the mechanisms ensuring the fidelity of chromosome segregation, which is essential for normal cell proliferation and embryonic development in humans.
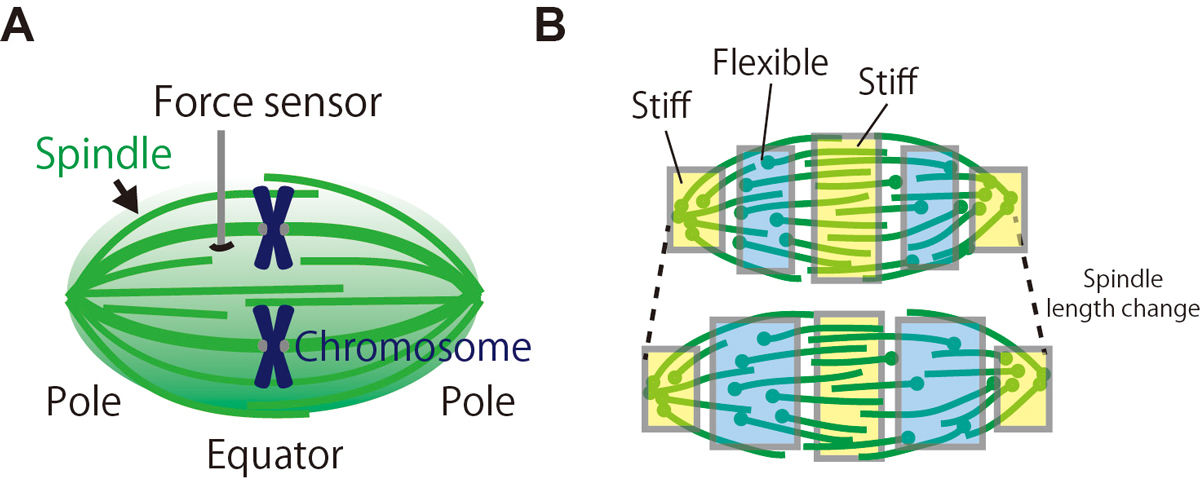
Figure: (A) The local mechanical architecture of the spindle was probed using a fiber-shaped microforce sensor. (B) The spindle was found to have a substantial mechanical heterogeneity (depicted in different colors), suggesting its robust yet adaptable nature required for error-free cell division.
Development of a CRISPR/Cas9-based tagging method and a degradation inhibitor for the auxin-inducible degron (AID) system
Generation of conditional auxin-inducible degron (AID) cells and tight control of degron-fused proteins using the degradation inhibitor auxinole
Aisha Yesbolatova, Toyoaki Natsume, Ken-ichiro Hayashi, Masato T. Kanemaki
Methods Available online 24 April 2019 DOI:10.1016/j.ymeth.2019.04.010
The auxin-inducible degron (AID) technology developed by the Kanemaki group is based on a plant-specific degradation pathway transplanted to non-plant cells. In human cells expressing a plant E3 ligase component, TIR1, a degron-fused endogenous protein is degraded within 15–45 min upon addition of the phytohormone auxin.
We demonstrated previously the generation of human HCT116 mutants in which the C terminus of endogenous proteins was fused with the degron by CRISPR–Cas9-based knock-in (Natsume et al. Cell Reports, 2016). In this study, A SOKENDAI student, Aisha Yesbolatova, developed new plasmids for N-terminal tagging and described a detailed protocol for the generation of AID mutants of human HCT116 and DLD1 cells (Figure 1).
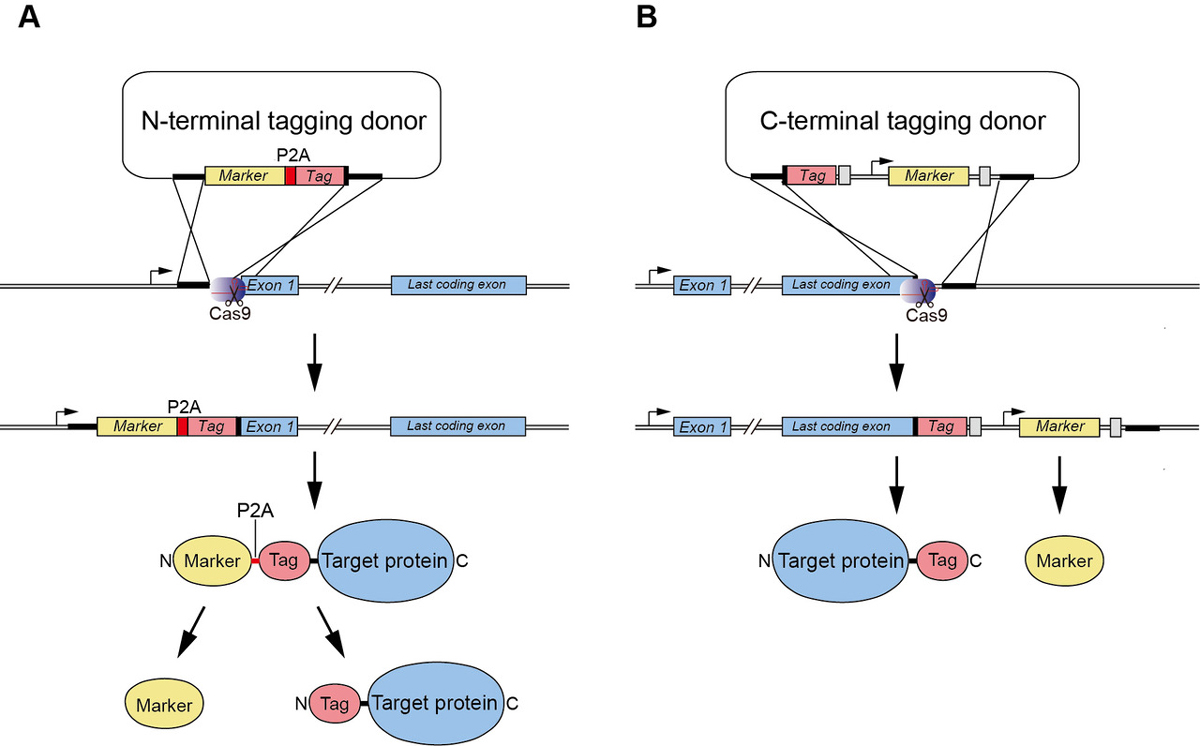
Figure1: (A) A new CRISPR/Cas9-based N-terminal tagging system. (B) C-terminal tagging.
Moreover, we report the use of a TIR1 inhibitor, auxinole, to suppress leaky degradation of degron-fused proteins (Figure 2). The addition of auxinole is also useful for rapid re-expression after depletion of degron-fused proteins.
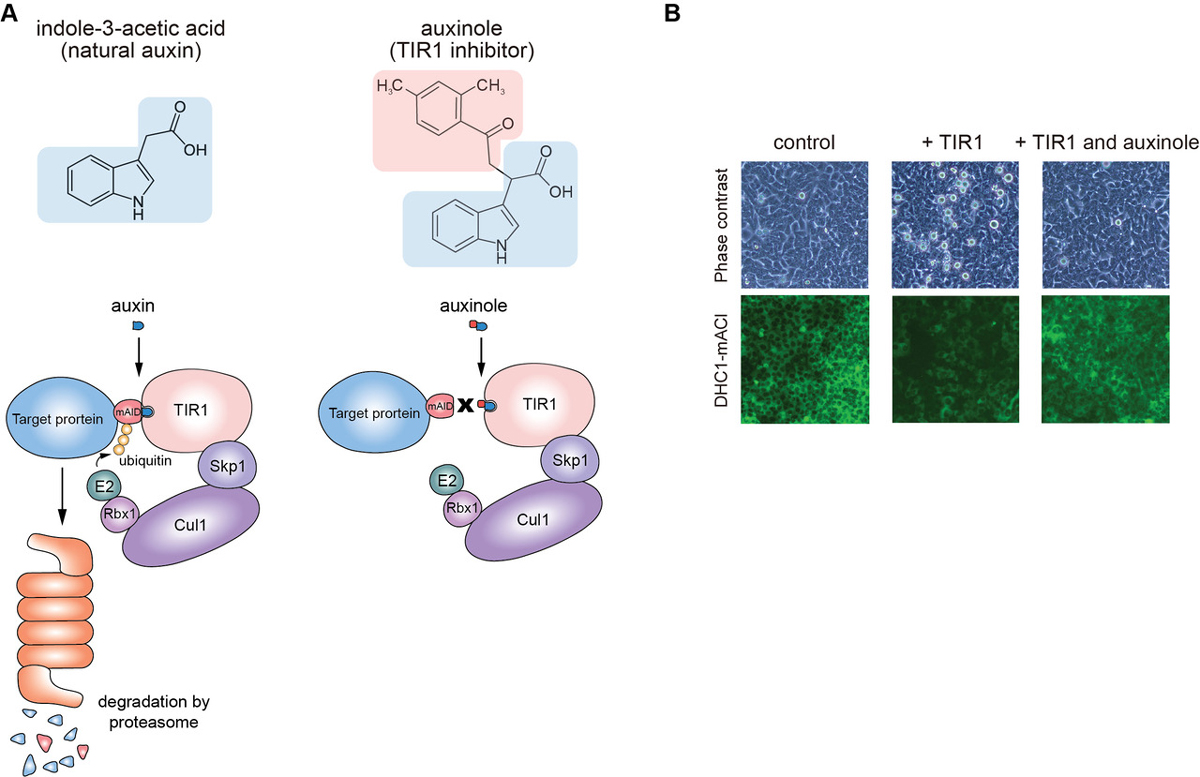
Figure2: (A) The structure of auxin and auxinole, and illustrations showing how these reagents work in the degradation pathway. (B) Suppression of leaky degradation in DHC1-tagged Tet-TIR1 cells. The cells were treated with only doxycycline to induce TIR1 expression or together with auxinole 48 h before microscopy.
We hope that these improvements enhance the utility of the AID technology for studying protein function in living human cells. All described plasmids are available from addgene and RIKEN-BRC.
This study was carried out as a collaboration with Prof. Ken-ichiro Hayashi of Okayama University of Science and was supported by JSPS KAKENHI (17K15068, 18H02170 and 18H04719), JST A-STEP (grant number AS2915150U), the Canon Foundation, the Asahi Glass Foundation, and the Takeda Science Foundation.
Evolutionary dynamics of developmental sequence in teleost lineage
Frequent nonrandom shifts in the temporal sequence of developmental landmark events during teleost evolutionary diversification
Fumihiro Ito, Tomotaka Matsumoto, Tatsumi Hirata
Evolution & Development 18 April 2019 DOI:10.1111/ede.12288
Development of multicellular organisms is characterized as a series of morphological events, whose temporal sequence, called as the developmental sequence, is well conserved in each individual species. In this study, we hypothesized that evolutionary changes in the sequence can be the basis for morphological diversifications, and examined the evolutionary dynamics of the developmental sequence in teleosts. Using the information from previous reports describing the development of 30 teleost species, we extracted the developmental sequences of 19 landmark events involving the formation of phylogenetically conserved body parts. Ancestral developmental sequences were then estimated by two different parsimony based methods. The phylogenetic comparisons of these sequences revealed that (1) the frequency of sequence changes differs among the events, (2) most of the sequence changes occurred as exchanges of temporally neighboring events, and (3) these changes in developmental sequences accumulate along the evolutionary time.
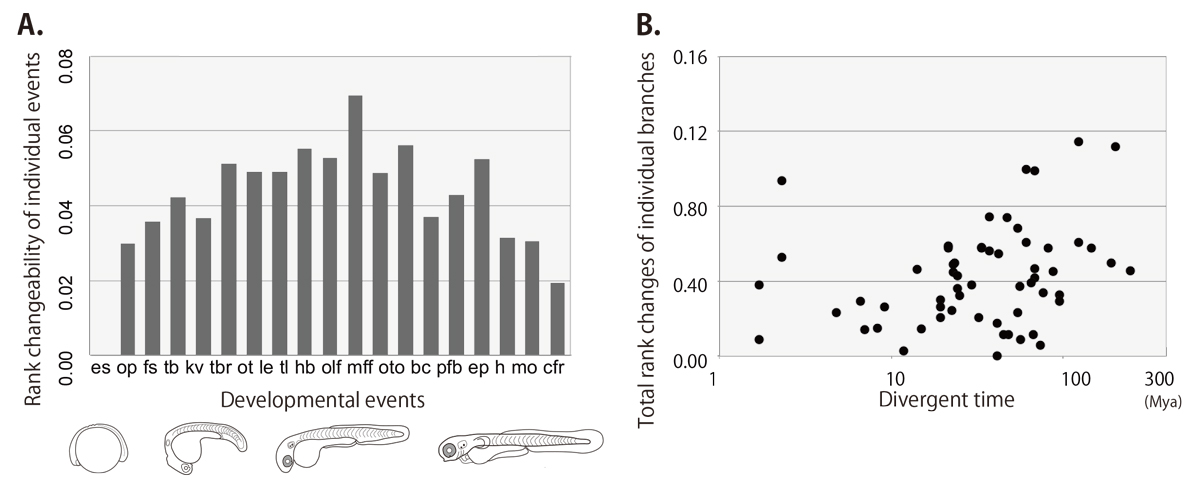
Figure: the rank changeability of individual developmental events (A) and the relationship between rank changes and divergent times (B) in teleost lineage estimated by the event-pairing method.















