Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
NIGINTERN 2017 Result Announcement
NIGINTERN 2017 Result Announcement
Identification of new cardiac progenitors
Mammalian Development Laboratory / Saga Group
Sfrp5 identifies murine cardiac progenitors for all myocardial structures except for the right ventricle
Fujii Masayuki, Akane Sakaguchi, Ryo Kamata, Masataka Nagao, Shilvia M. Evans, Masao Yoshizumi, Akihiko Shimono, Yumiko Saga, and Hiroki Kokubo
Nature Communications. DOI:10.1038/NCOMMS1466
Upon acquirement of pulmonary circulation, the ancestral heart may have been remodelled coincidently with, or accompanied by, the production and rearrangement of progenitor cells. However, the progenitor populations that give rise to the left ventricle (LV) and sinus venosus (SV) are still ambiguous. Here we show that the expression of Secreted frizzled-related protein Sfrp5 in the mouse identifies common progenitors for the outflow tract (OFT), LV, atrium and SV but not the right ventricle (RV). Sfrp5 expression begins at the lateral sides of the cardiac crescent, excluding early differentiating regions, and continues in the venous pole, which gives rise to the SV. Lineage-tracing analysis revealed that descendants of Sfrp5-expressing cells at E7.5 contribute not only to the SV but also to the LV, atria and OFT and are found also in the dorsal splanchnic mesoderm accompanied by the expression of the secondary heart field marker, Islet1. These findings provide insight into the arrangement of cardiac progenitors for systemic circulation. This study was conducted as a collaboration research with Dr. Kokubo who used to be an assistant professor in Mammalian Development Lab and currently a lecturer of Hiroshima University.
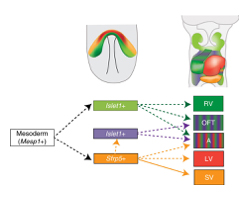
Upper panels show the expression area ofSfrp5 (orange) andIslet1 (light green) at E7.5 (left) and the distribution of descendant cells at E9.5 (right).
The lower panel shows the lineage contribution of the heart. Mesp1-positive mesodermal cells are common cardiac precursors.TransientIslet1-expressing cells that do not expressSfrp5 contribute to the RV, OFT and atrium (green). Sfrp5-positive cells contribute to the LV, atrium and OFT (orange) the SV
Yuki TUCHIYA (KITAGAWA lab) and Kazunori YAMAMOTO (KIMURA lab) received Morishima Awards
On March 14, 2017 two students who graduated in March 2016 received Morishima Awards from the Department of Genetics, SOKENDAI. The Morishima Award was established using a donation from the Morishima family honoring the late professor emeritus of NIG, Dr. Hiroko MORISHIMA. It awards students who receive a doctoral degree from the Department of Genetics to acknowledge their outstanding performances during PhD studies.
・Yuki TSUCHIYA (Division of Centrosome Biology, thesis advisor Daiju KITAGAWA)
thesis title : The role of Cep295 in centrosome biogenesis of human cells
・Kazunori YAMAMTO (Cell Architecture Laboratory, thesis advisor: Akatsuki KIMURA)
thesis title : A study on inter-cellular forces for blastomere configurations in developing embryos

Endoplasmic Reticulum-Mediated Microtubule Alignment Governs Cytoplasmic Streaming
Press release
Endoplasmic Reticulum-Mediated Microtubule Alignment Governs Cytoplasmic Streaming
Kenji Kimura, Alexandre Mamane, Tohru Sasaki, Kohta Sato, Jun Takagi, Ritsuya Niwayama, Lars Hufnagel, Yuta Shimamoto, Jean-François Joanny, Seiichi Uchida, and Akatsuki Kimura
Nature Cell Biology (2017) DOI:10.1038/ncb3490
Pressrelease (In Japanese only)
A research group lead by Drs. Kenji Kimura and Akatsuki Kimura at NIG revealed the self-organization mechanism of cytoplasmic streaming in Caenorhabditis elegans zygotes. Cytoplasmic streaming refers to a collective movement of cytoplasm observed in many cell types. The mechanism of meiotic cytoplasmic streaming (MeiCS) in C. elegans zygotes was puzzling as the direction of the flow is not predefined by cell polarity and occasionally reverses. The research group demonstrated that the endoplasmic reticulum (ER) network structure is required for the collective flow (Fig. 1). Using a combination of RNAi, microscopy, and image processing of C. elegans zygotes, the group devised a theoretical model, which reproduced and predicted the emergence and reversal of the flow. They proposed a positive feedback mechanism, where a local flow generated along a microtubule is transmitted to neighboring regions through the ER (Fig. 2). This, in turn, aligns microtubules over a broader area to self-organize the collective flow. The proposed model could be applicable to various cytoplasmic streaming phenomena in the absence of predefined polarity. The increased mobility of cortical granules by MeiCS correlated with the efficient exocytosis of the granules to protect the zygotes from osmotic and mechanical stresses.
The research was conducted as collaboration between NIG (Cell Architecture lab and Quantitative Mechanobiology lab), Kyushu University, Institut Curie (France) and EMBL (Germany).
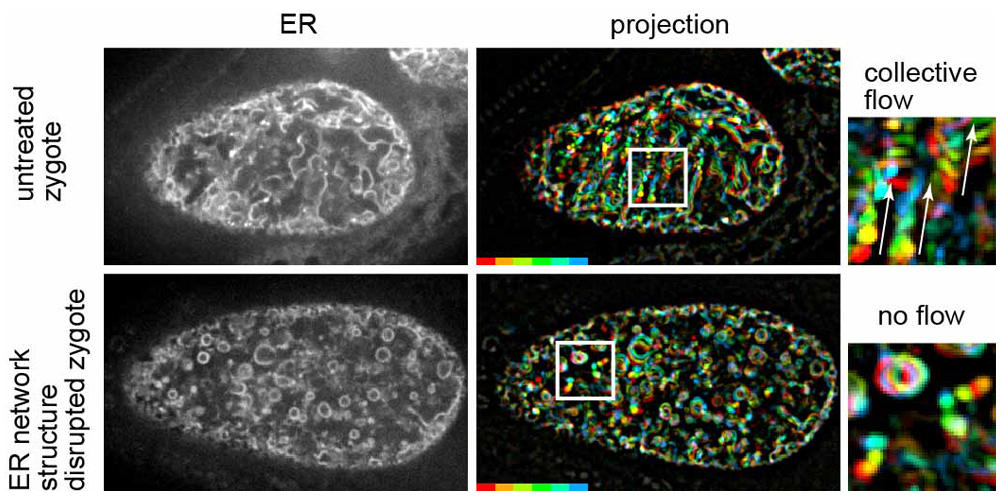
Fig. 1
Left panels show fluorescence confocal images of the ER during MeiCS in the C. elegans zygote (upper: untreated zygote, bottom: RNAi-treated zygote in which the network structure of the ER was fragmented). Middle panels show projections of sequential images of the ER. Fluorescence signals are colored to indicate their trajectories. Fragmentation of the ER network inhibited the flow. Boxed region is magnified on the right. White arrows indicate the flow direction.
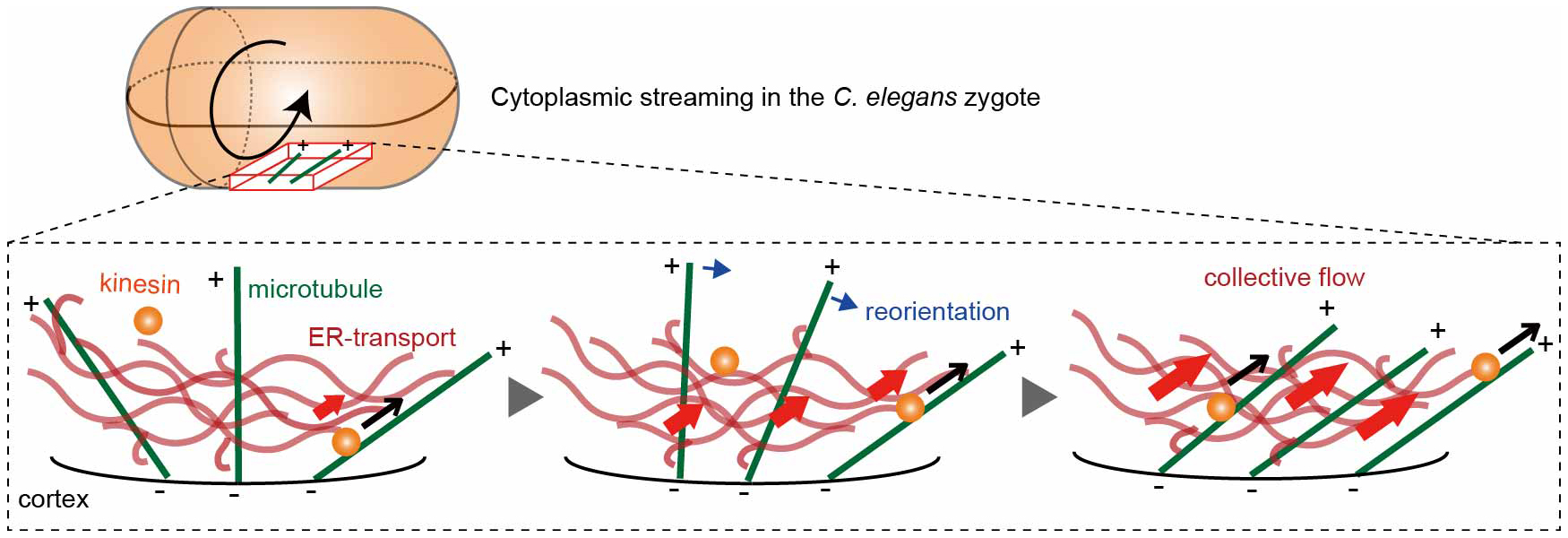
Fig. 2
ER-mediated positive feedback model. When kinesin transports the ER network along a cortical microtubule (black arrows), neighboring microtubules are biased to reorient (blue arrows) toward the ER motion, enhancing collective flow (red arrows). (−) and (+) signs indicate microtubule polarity.
PIGN prevents protein aggregation in the ER
Multicellular Organization Laboratory / Sawa Group
PIGN prevents protein aggregation in the endoplasmic reticulum independently of its function in the GPI synthesis
Shinji Ihara, Sohei Nakayama, Yoshiko Murakami, Emiko Suzuki, Masayo Asakawa, Taroh Kinoshita and Hitoshi Sawa
J Cell Sci 2017 130: 602-613; DOI:10.1242/jcs.196717
1. Protein aggregation is a common feature of many neurodegenerative diseases and often results from defective folding processes. We found that PIGN functions in protein quality control to prevent protein aggregation within the ER in C. elegans and in mammalian cells. Although PIGN was originally identified as an enzyme involved in glycosylphosphatidylinositol (GPI)-anchor biosynthesis, we show that the function of PIGN in protein quality control is independent of its enzymatic activities. In human, several mutations in PIGN have been shown to cause multiple congenital anomalies-hypotonia-seizures syndrome 1 (MCAHS1) that shows dysmorphic facial features and Fryns syndrome that cause lethality in the neonatal period. C. elegans with pign mutations created by CRISPR/Cas9 that correspond to MCAHS1 patients also cause protein aggregation in the ER, implying that the dysfunction of the PIGN non-canonical function might affect symptoms of MCAHS1 and potentially those of other diseases.
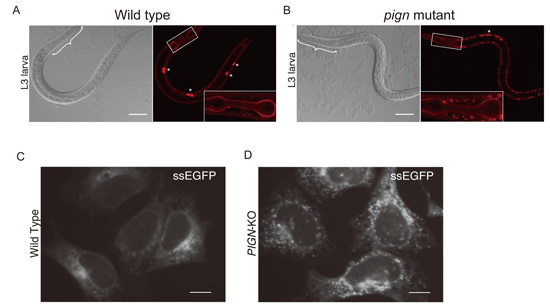
Accumulation of secretory proteins in C. elegans and mammalian cells with PIGN mutations.
(A) The localization of collagen-IV::mCherry (right) and differential interference contrast (DIC) images (left) of wild-type (A) and PIGN mutant (B) C. elegans.
The localization of secreted soluble EGFP (ssEGFP) in wild-type (C) and PIGN-knockout (D) HEK293 cells.
Three new assistant professors join NIG
New assistant professors join NIG as of March 1, 2017.
- Satoko YOSHIBA: Division of Centrosome Biology • Kitagawa Group
- Misuzu TAKAHASHI: Plant Genetics Laboratory • Sato Group
- Takeshi KAWASHIMA: Laboratory of Biological Networks • Arita Group















