A new method of rapid protein depletion in nematode individuals using an improved auxin-inducible degron system, AID2
The auxin-inducible degron 2 (AID2) system enables controlled protein knockdown during embryogenesis and development in Caenorhabditis elegans.
Negishi T#, Kitagawa S#, Horii N, Tanaka Y, Haruta N, Sugimoto A, Sawa H, Hayashi KI, Harata M*, Kanemaki MT*.
# These authors contributed equally * Co-corresponding authors
Genetics (2022) 220, iyab218 DOI:10.1093/genetics/iyab218
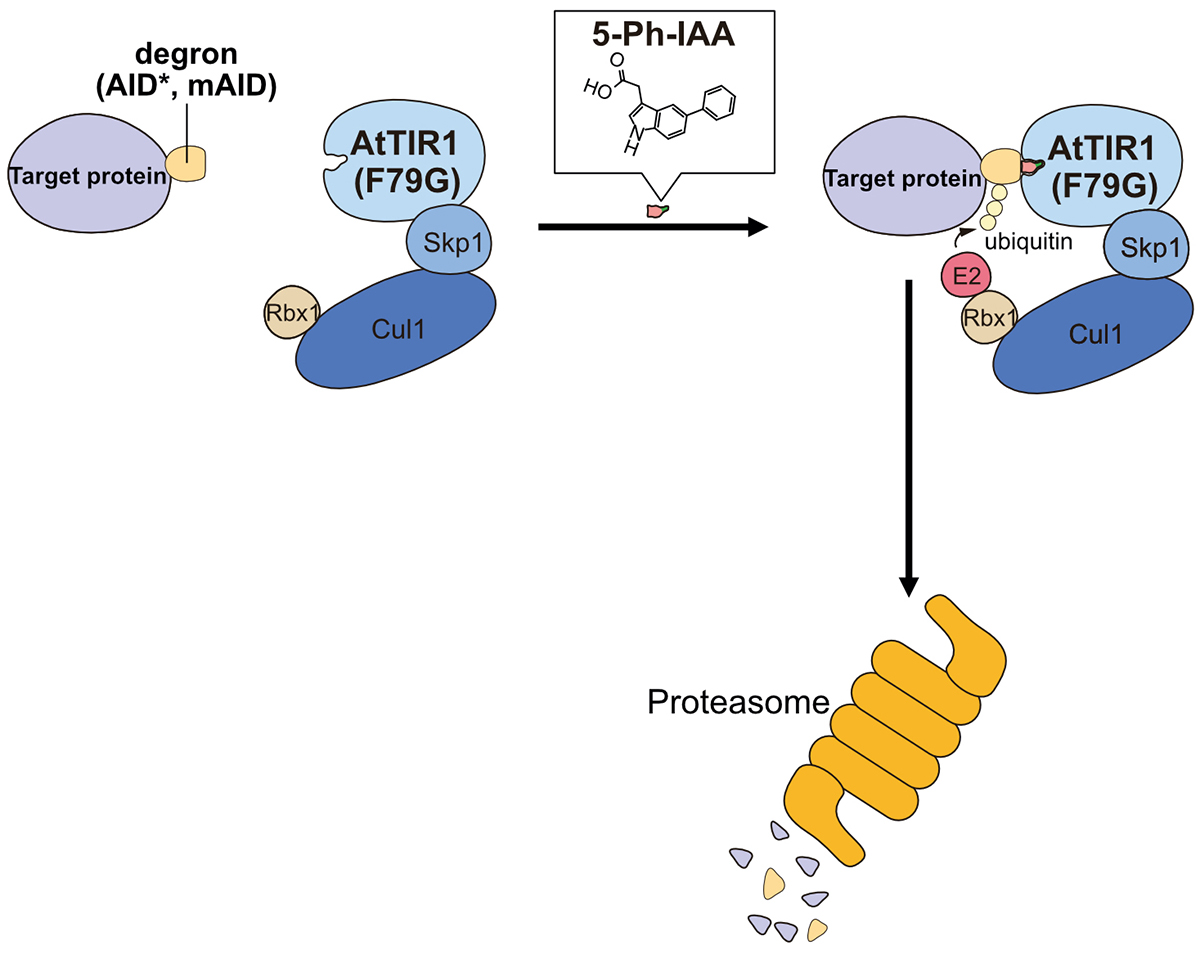
To analyze the protein function of C. elegans, it is useful to study the phenotype by suppressing the protein function. For this purpose, nematode individuals with mutated genes and the RNA interference method have been used. However, genes that play an essential role in nematode development may cause a developmental defect due to the loss of the gene, making further analysis difficult. In addition, due to a large amount of maternally-derived mRNA and proteins in the eggs, the functions of proteins normally involved in early development may not be expressed as a phenotype in early development using existing techniques. The auxin-inducible degron (AID) system that we have established rapidly degrades and removes target proteins at any given time and allows us to observe phenotypes that cannot be seen using existing techniques. The AID method has already been applied to C. elegans by a group in the U.S. and is now being used in various studies. However, the conventional AID method has some problems, such as leaky target degradation in the absence of auxin and the effects of high concentrations of auxin.
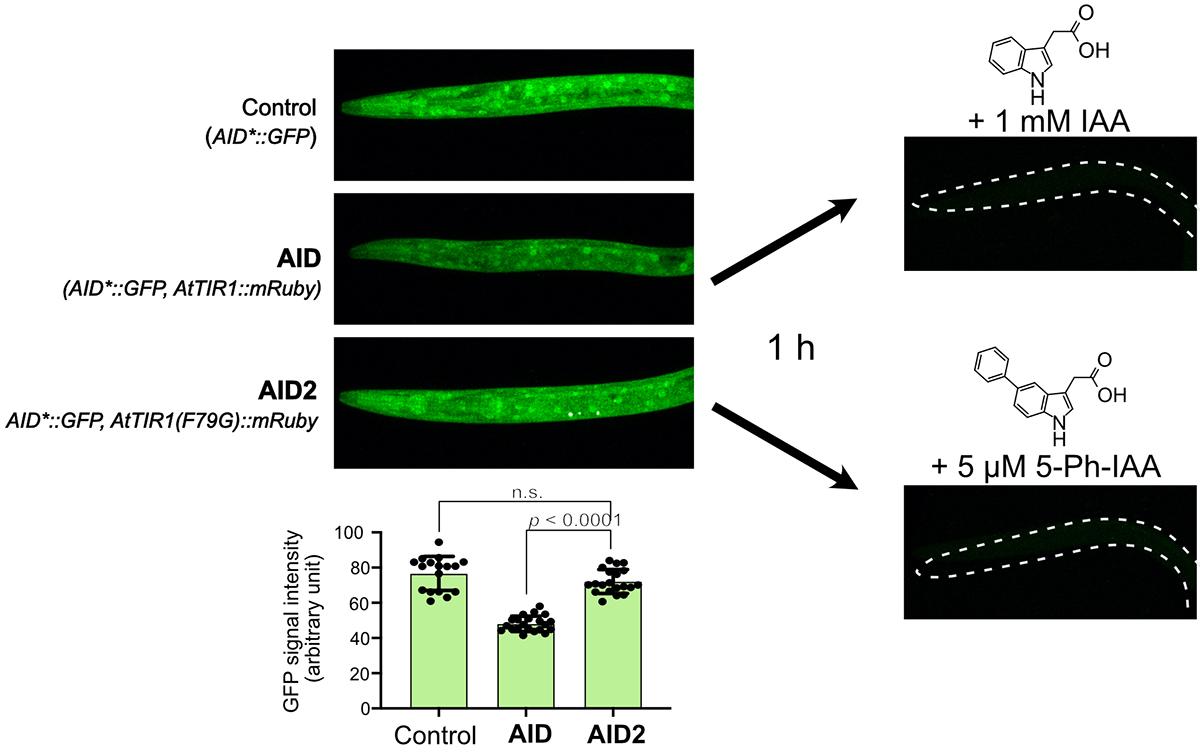
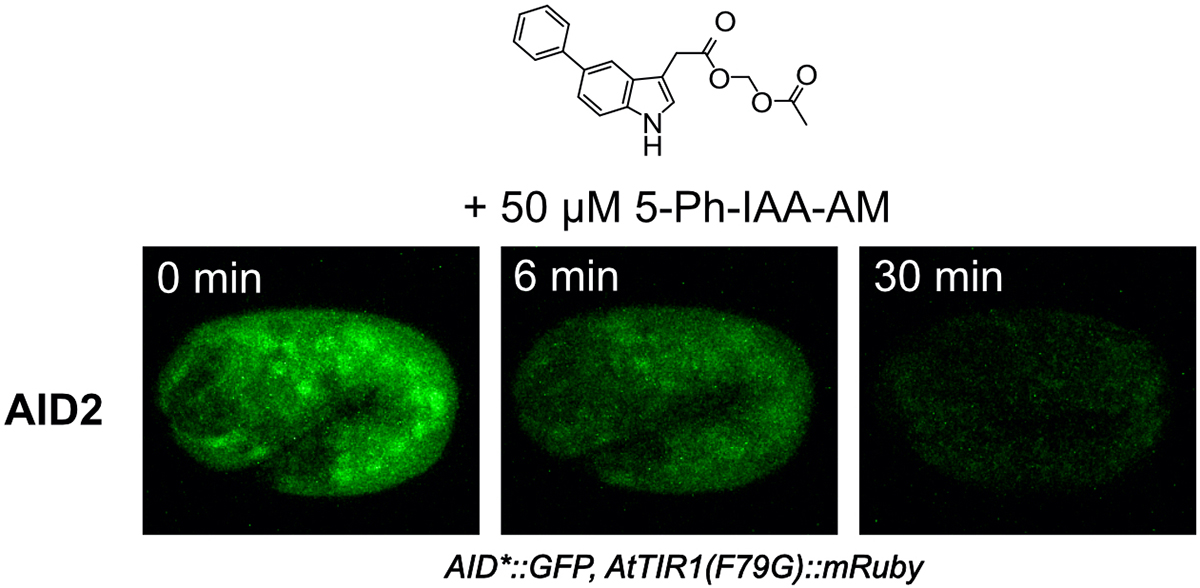
Therefore, we applied an improved method, AID2, which we developed last year using budding yeast, cultured cells, and mice, to C. elegans to overcome these problems (Figure 1). As a result, we found that AID2 completely suppressed the leaky degradation in C. elegans and rapidly induced target degradation with 1/1300 of the ligand concentration (Figure 2). Furthermore, by degrading the histone H2A.Z protein, we succeeded in observing a developmental defect that had not been reported previously. Furthermore, to induce degradation in the embryo in the egg, we developed a modified ligand suitable for eggshell permeabilization, enabling rapid proteolysis in the embryo (Figure 3).
This research was led by Assistant Professor Takefumi Negishi and Professor Masato Kanemaki at the National Institute of Genetics, and a graduate student Saho Kitagawa and Professor Masahiko Harada at Tohoku University, in collaboration with Professor Kenichiro Hayashi at Okayama University of Science, Professor Hitoshi Sawa at the National Institute of Genetics, and Professor Asako Sugimoto at Tohoku University.