Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
ROS production at micrometer-scale precision by receptor signaling
SCHENGEN receptor module drives localized ROS production and lignification in plant roots
Satoshi Fujita, Damien De Bellis, Kai H Edel, Philipp Köster, Tonni Grube Andersen, Emanuel Schmid-Siegert, Valérie Denervaud Tendon, Alexander Pfister, Peter Marhavý, Robertas Ursache, Verónica G. Doblas, Marie Barberon, Jean Daraspe, Audrey Creff, Gwyneth Ingram, Jörg Kudla, Niko Geldner
EMBO J (2020)e103894 DOI:10.15252/embj.2019103894
Reactive oxygen species (ROS) impact many physiological processes in animals or plants, but its production by NADPH oxidases is strictly regulated because of extremely high reactivity. Many plant receptor pathways are known as critical regulators of ROS production, but how they spatially control ROS production is yet to be clarified.
Fujita et al. (2020) established a phospho-signaling pathway that integrates direct, rapid activation of ROS production with positional information and transcriptional changes to form proper diffusion barriers. Firstly, the authors presented a direct connection from a peptide-receptor complex to NADPH oxidases via a membrane-anchored kinase by biochemically. Next, the authors focused on the membrane-anchored kinase that localizes on the plasma membrane in a polar fashion. Manipulation of the kinase localization successfully showed that this biased distribution of the kinase protein gave the positional cue, which enables the SCHENGEN pathway to activate only one side of the Casparian strip at micrometer-scale precision. This work would contribute to highlight how other receptor pathways could control local ROS production.
This work was mainly done by Dr. Satoshi Fujita (former postdoctoral fellow in University of Lausanne, currently NIG research fellow) in Prof. Niko Geldner’s group with a collaboration with the Central imaging, Electron microscope, and Genomics facilities of University of Lausanne, Swiss Institute of bioinformatics, Prof. Jörg Kudla’s group at University of Munster and Dr. Gwyneth Ingram’s group at University of Lyon.
This work was aided by grants no. 31003A_156261 and 310030E_176090 (N.G.), from the Swiss National Science Foundation, an ERC Consolidator Grant (616228-ENDOFUN) (N.G.), DFG grant (Ku931/14-1 (J.K.)) FEBS long-term fellowship (P.M.), EMBO long-term fellowship (R.U., M.B.), Marie Curie postdoctoral fellowship (T.G.A,), Fundacion Alfonso Martin Escudero fellowship (V.G.D.), and a Japan Society for the Promotion of Science (JSPS) fellowship (S.F.).
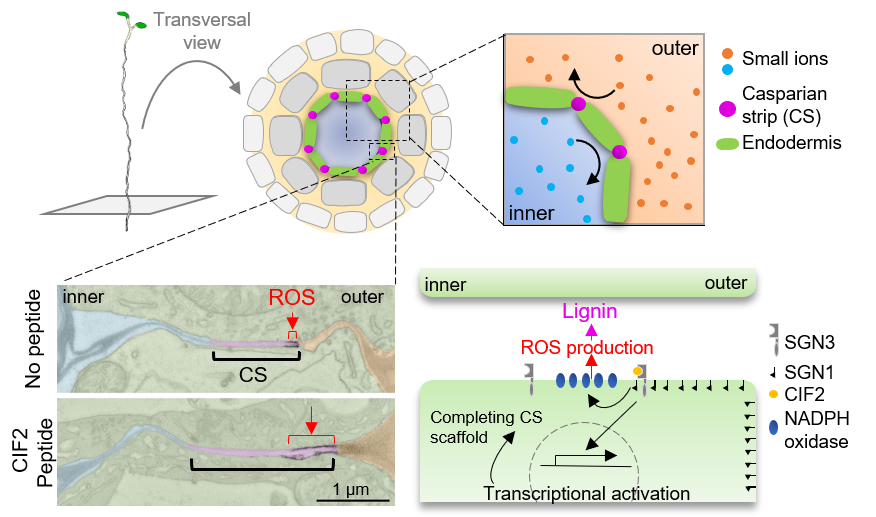
Figure: Plant roots have a lignin-based diffusion barrier, namely Casparian strips. Localized ROS production (EM pictures) by asymmetrical signal activation (schematic model) sustains functional barrier formation.
- RNA seq data(transcriptional changes after CIF peptide treatment)
Gastrointestinal Neurons Expressing HCN4 Regulate Retrograde Peristalsis
Gastrointestinal Neurons Expressing HCN4 Regulate Retrograde Peristalsis
Kensuke Fujii, Koichi Nakajo, Yoshihiro Egashira, Yasuhiro Yamamoto, Kazuya Kitada, Kohei Taniguchi, Masaru Kawai, Hideki Tomiyama, Koichi Kawakami, Kazuhisa Uchiyama, and Fumihito Ono
Cell Reports 30(9), 2879-2888 (2020). DOI:10.1016/j.celrep.2020.02.024
Peristalsis is indispensable for physiological function of the gut. The enteric nervous system (ENS) plays an important role in regulating peristalsis. While the neu- ral network regulating anterograde peristalsis, which migrates from the oral end to the anal end, is charac- terized to some extent, retrograde peristalsis re- mains unresolved with regards to its neural regula- tion. Using forward genetics in zebrafish, we reveal that a population of neurons expressing a hyperpo- larization-activated nucleotide-gated channel HCN4 specifically regulates retrograde peristalsis. When HCN4 channels are blocked by an HCN channel inhibitor or morpholinos blocking the protein ex- pression, retrograde peristalsis is specifically attenu- ated. Conversely, when HCN4(+) neurons expressing channelrhodopsin are activated by illumination, retrograde peristalsis is enhanced while anterograde peristalsis remains unchanged. We propose that HCN4(+) neurons in the ENS forward activating sig- nals toward the oral end and simultaneously stimu- late local circuits regulating the circular muscle.
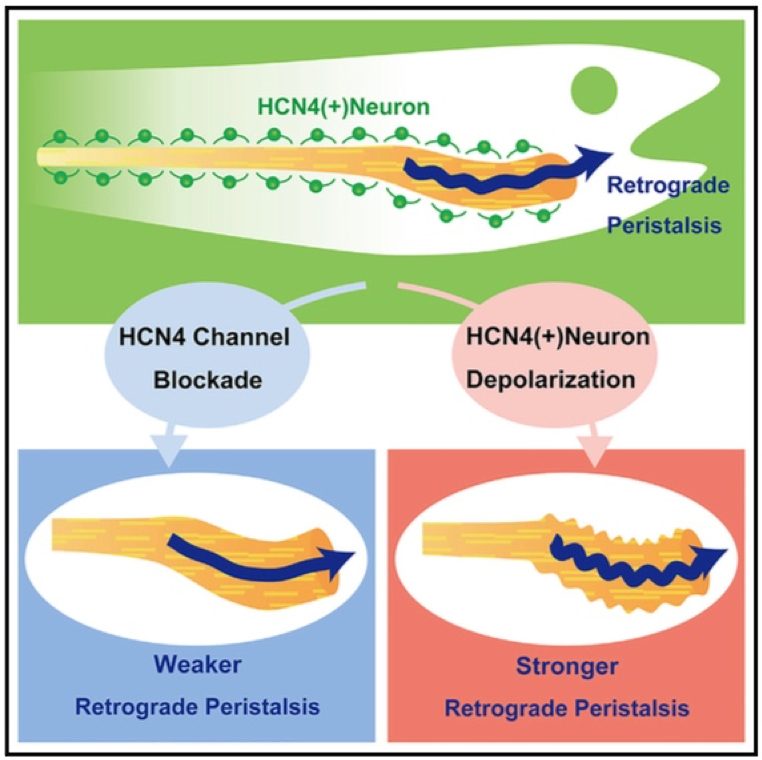
Figure1: Gastrointestinal neurons expressing a hyperpolarization-activated nucleotide- gated channel HCN4 regulate retrograde peristalsis, which migrates from the anal end to the oral end of the gut.
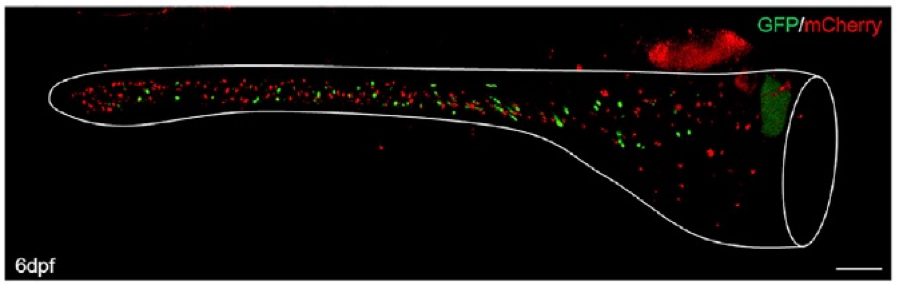
Figure2: Visualization of neurons (red) and HCN4-expressing neurons (green) in the zebrafish intestine.
A virtual reality system to analyze neural activity and behavior in adult zebrafish
Press release
A virtual reality system to analyze neural activity and behavior in adult zebrafish
Kuo-Hua Huang, Peter Rupprecht, Thomas Frank, Koichi Kawakami, Tewis Bouwmeester and Rainer W. Friedrich
Nature methods 02 March 2020 DOI:10.1038/s41592-020-0759-2
Press release (In Japanese only)
Virtual realities are powerful tools to analyze and manipulate interactions between animals and their environment and to enable measurements of neuronal activity during behavior. In many species, however, optical access to the brain and/or the behavioral repertoire are limited. We developed a high-resolution virtual reality for head-restrained adult zebrafish, which exhibit cognitive behaviors not shown by larvae. We noninvasively measured activity throughout the dorsal telencephalon by multiphoton calcium imaging. Fish in the virtual reality showed regular swimming patterns and were attracted to animations of conspecifics. Manipulations of visuo-motor feedback revealed neurons that responded selectively to the mismatch between the expected and the actual visual consequences of motor output. Such error signals were prominent in multiple telencephalic areas, consistent with models of predictive processing. A virtual reality system for adult zebrafish therefore provides opportunities to analyze neuronal processing mechanisms underlying higher brain functions including decision making, associative learning, and social interactions.
Source: Kuo-Hua Huang , et al., Published: 02 March 2020
DOI: 10.1038/s41592-020-0759-2
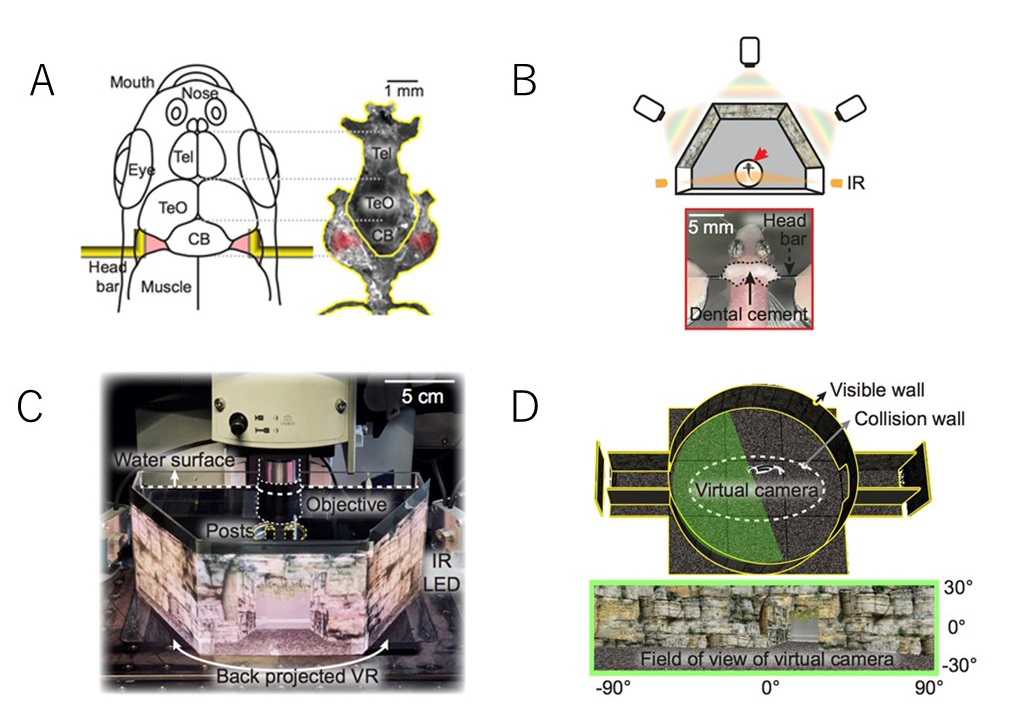
Fig: Virtual reality system to analyze neural activity and behavior in adult zebrafish
A: Head fixation with L-shaped bars
B: Virtual reality projected by three projectors onto a panoramic screen
C: Setup for virtual reality and two-photon imaging
D: Virtual reality seen by the head-fixed zebrafish
- This study is based on the previous study.















