Microfocus X-ray CT (microCT) Imaging of Actinia equina (Cnidaria), Harmothoe sp. (Annelida), and Xenoturbella japonica (Xenacoelomorpha).
Microfocus X-ray CT (microCT) Imaging of Actinia equina (Cnidaria), Harmothoe sp. (Annelida), and Xenoturbella japonica (Xenacoelomorpha).
Maeno A, Kohtsuka H, Takatani K, Nakano H.
Journal of Visualized Experiments 150 e59161 1-9 DOI:10.3791/59161
Traditionally, biologists have had to rely on destructive methods such as sectioning in order to investigate the internal structures of opaque organisms. Non-destructive microfocus X-ray computed tomography (microCT) imaging has become a powerful and emerging protocol in biology, due to technological advancements in sample staining methods and innovations in microCT hardware, processing computers, and data analysis software. However, this protocol is not commonly used, as it is in the medical and industrial fields. One of the reasons for this limited use is the lack of a simple and comprehensible manual that covers all of the necessary steps: sample collection, fixation, staining, mounting, scanning, and data analyses. Another reason is the vast diversity of metazoans, particularly marine invertebrates. Because of marine invertebrates’ diverse sizes, morphologies, and physiologies, it is crucial to adjust experimental conditions and hardware configurations at each step, depending on the sample. Here, microCT imaging methods are explained in detail using three phylogenetically diverse marine invertebrates: Actinia equina (Anthozoa, Cnidaria), Harmothoe sp. (Polychaeta, Annelida), and Xenoturbella japonica (Xenoturbellida, Xenacoelomorpha). Suggestions on performing microCT imaging on various animals are also provided.
Source: Maeno A. et al, (2019) J Vis Exp. 6 Augst, DOI:10.3791/59161.
Inquiries : Technical Specialist, MAENO, Akiteru(amaeno@nig.ac.jp)
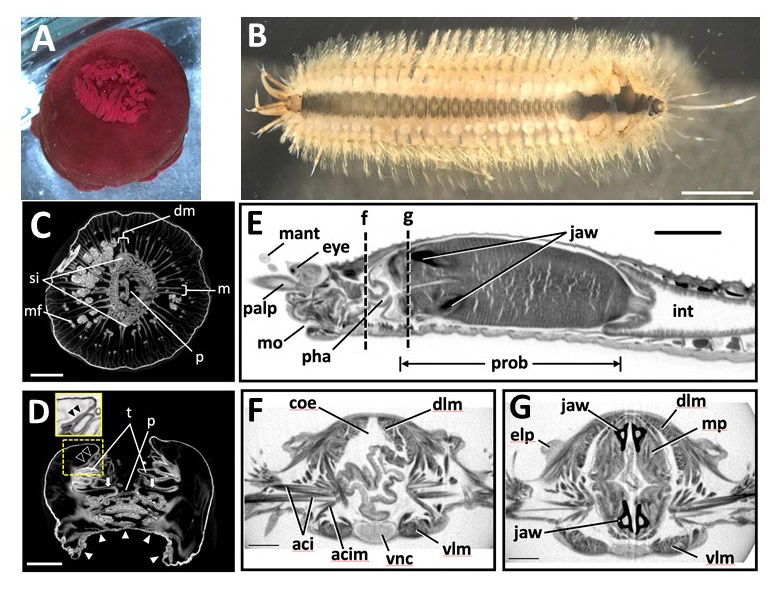
Figure: Marine invertebrate animals observed in this study. (A,B)
(A) Live anesthetized Actinia equina (Anthozoa, Cnidaria).
(B) Live anesthetized Harmothoe sp. (Polychaeta, Annelida).Most of the elytra were already missing at this stage, with only four remaining near the posterior end. Scale bars = 3 mm.
Scanned and reconstructed images of marine invertebrates. (C-G)
(C) Transverse and (D) longitudinal sections of Actinia equina. Scale bars in C, D = 3 mm.
(E-G) Harmothoe sp. (Polychaeta, Annelida). (E) Sagittal section of the anterior part. (F, G) Transverse section at the dotted lines f and g in (E). Scale bars: E = 1 mm; F, G = 0.3 mm.
Volume rendering image and transverse section movie of the whole body of Harmothoe sp. 6 sec to 16 sec: 3D volume rendering image. Top: left view, bottom: frontal view. 17 sec to 1min 42 sec: Transverse section movie. The position of the section is shown with a moving green line on the sagittal section image at top. From 17 sec to 53 sec, transverse section movie of the anterior part of the specimen generated from Pinpoint Scan is shown at the left bottom. From 1 min 7 sec to 1 min 14 sec, a 3D model made using Imaris software showing the positions of major organs is present at right top. Anatomical supervision: Masaatsu Tanaka (Kagoshima University)
This technique is one of the bases of following researches.
・ Evolution of Shh endoderm enhancers during morphological transition from ventral lungs to dorsal gas bladder
・ Regulation of internode patterning and vein anastomosis in maize stems.
・ Combination of multiple Shh enhancers controls tooth development in mouse
・Development of organs in living whole embryo/larval grafts in zebrafish
・ Enhancer adoption changes limb morphology
・When do male and female differences appear in the development of beetle horns? (National Institute for Basic Biology )
・A mouse model of human chromosomal disorder, partial trisomy distal 4q















