Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Scaffolding system for three-dimensional organization of neurons in the developing neocortex
Press release
Memo1 Mediated Tiling of Radial Glial Cells Facilitates Cerebral Cortical Development
Naoki Nakagawa, Charlotte Plestant, Keiko Yabuno-Nakagawa, Jingjun Li, Janice Lee, Chu-Wei Huang, Amelia Lee, Oleh Krupa, Aditi Adhikari, Suriya Thompson, Tamille Rhynes, Victoria Arevalo, Jason L. Stein, Zoltán Molnár, Ali Badache, E. S. Anton
Neuron Published:July 02, 2019 DOI:10.1016/j.neuron.2019.05.049
Press release (In Japanese only)
In the mammalian neocortex, formation of distinct neuronal layers is important for precise information processing. During the embryonic brain development, neural progenitor cells not only generate neurons, but also play an important role in the neuronal layer formation. The process sprouted from the neural progenitor cell, termed radial process (Figure 1), is used as “guide” for migrating neurons. Each neural progenitor cell has a single radial process that extends toward the brain surface in a non-overlapping, regularly interspaced manner, therefore functioning as a “scaffolding system” for the neuronal migration and layer formation. However, the molecular and cellular mechanisms for the generation of this scaffolding system is not well understood.
A team of Dr. Naoki Nakagawa at the National Institute of Genetics and Dr. Eva S. Anton at the University of North Carolina School of Medicine identified mediator of cell motility 1 (Memo1) as a key gene for building this scaffolding system and revealed its role in the neuronal layer formation in the mouse neocortex.
By analyzing embryonic brains of Memo1-knockout mice, they found that radial processes of Memo1-deficient neural progenitor cells showed extensive branching and altered spatial distribution. These defects disrupted the scaffolding system of neural progenitor cells and resulted in neuronal misplacement and disorganized layers. These findings demonstrate that Memo1 is important for neural progenitor cells to form the regularly interspaced scaffolding system to organize cortical neurons into distinct neuronal layers (Figure 1).
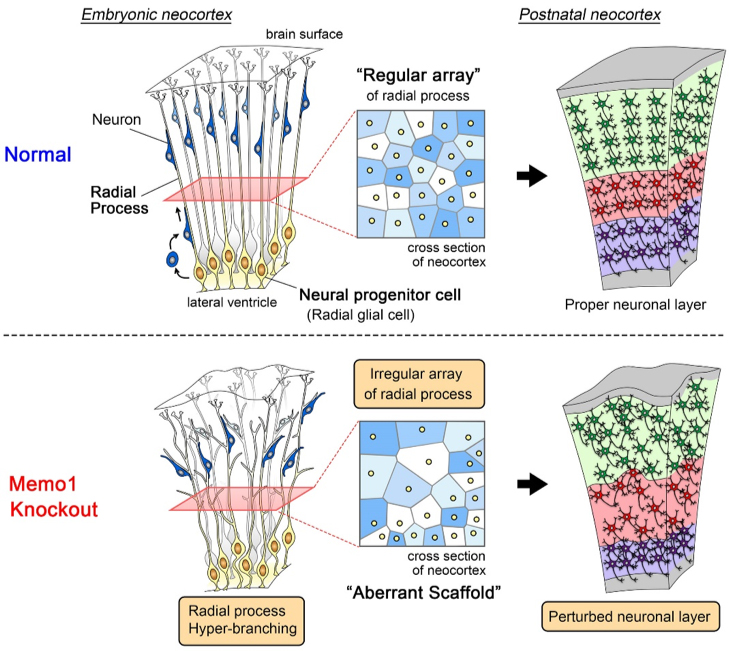
Figure 1: The scaffolding system of neural progenitor cells and neuronal layer formation
(Top) In the embryonic neocortex, neural progenitor cells are located at the ventricular surface and extend single radial processes toward the brain surface. Radial processes do not overlap with each other and are regularly interspaced. Newborn neurons migrate toward the superficial area by using this regular array of radial process as a “scaffold”, eventually forming the proper neuronal layer.
(Bottom) In the Memo1-knockout mouse neocortex, the absence of Memo1 function in neural progenitor cells causes hyper-branching and irregular distribution of radial processes. These phenotypes generate an aberrant scaffold, leading to perturbed neuronal layer formation.
Summer Holiday (Aug. 15,16)
NIG will be closed from August 15 and August 16, 2019 for summer holiday.
Thank you for your understanding and cooperation.
“Safe” cellular proliferation depending on “toxic” photosynthesis
Day/Night Separation of Oxygenic Energy Metabolism and Nuclear DNA Replication in the Unicellular Red Alga Cyanidioschyzon merolae.
Shin-ya Miyagishima, Atsuko Era, Tomohisa Hasunuma, Mami Matsuda, Shunsuke Hirooka, Nobuko Sumiya, Akihiko Kondo, Takayuki Fujiwara
mBio 10(4), e00833-19, 2019 DOI:10.1128/mBio.00833-19
Eukaryotes acquired chloroplasts through an endosymbiotic event in which a cyanobacterium or a unicellular eukaryotic alga was integrated into a previously nonphotosynthetic eukaryotic cell. Photosynthesis by chloroplasts enabled algae to expand their habitats and led to further evolution of land plants. However, photosynthesis causes greater oxidative stress than mitochondrion-based respiration. In seed plants, cell division is restricted to nonphotosynthetic meristematic tissues and populations of photosynthetic cells expand without cell division. Thus, seemingly, photosynthesis is spatially sequestrated from cell proliferation. In contrast, eukaryotic algae possess photosynthetic chloroplasts throughout their life cycle. Here we show that oxygenic energy conversion (daytime) and nuclear DNA replication (night time) are temporally sequestrated in C. merolae. This sequestration enables “safe” proliferation of cells and allows coexistence of chloroplasts and the eukaryotic host cell, as shown in yeast, where mitochondrial respiration and nuclear DNA replication are temporally sequestrated to reduce the mutation rate.
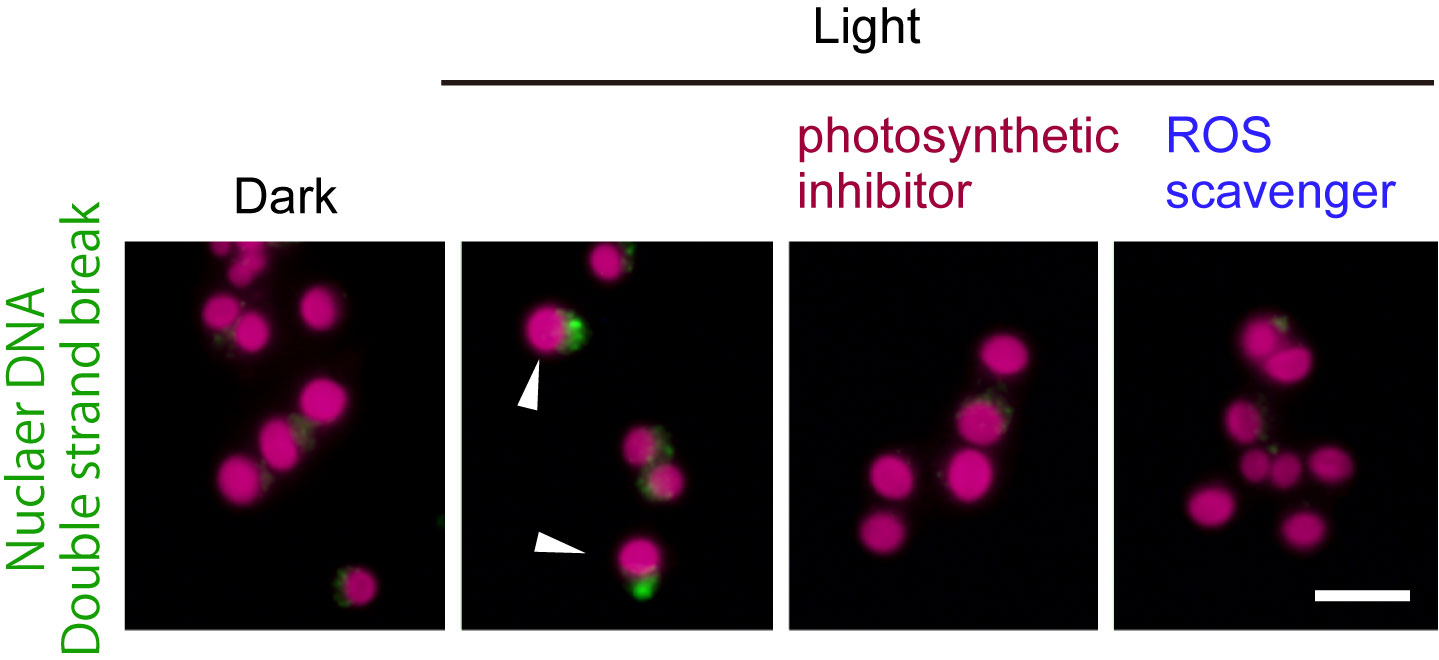
Figure1: The unicellular red alga Cyanidioschyzon merolae was cultured under a 12-h light / 12-h dark cycle. The cells early in the dark period (dark) were illuminated (light) either with or without DCMU (photosynthetic inhibitor) or TEMPOL (ROS scavenger). The green fluorescence indicates accumulation of MRE11 in the nucleus (DNA double strand break). The red is autofluorescence of the chloroplast. When the cells during the subjective night perform photosynthesis, the frequency of nuclear DSB (arrowheads) increases.
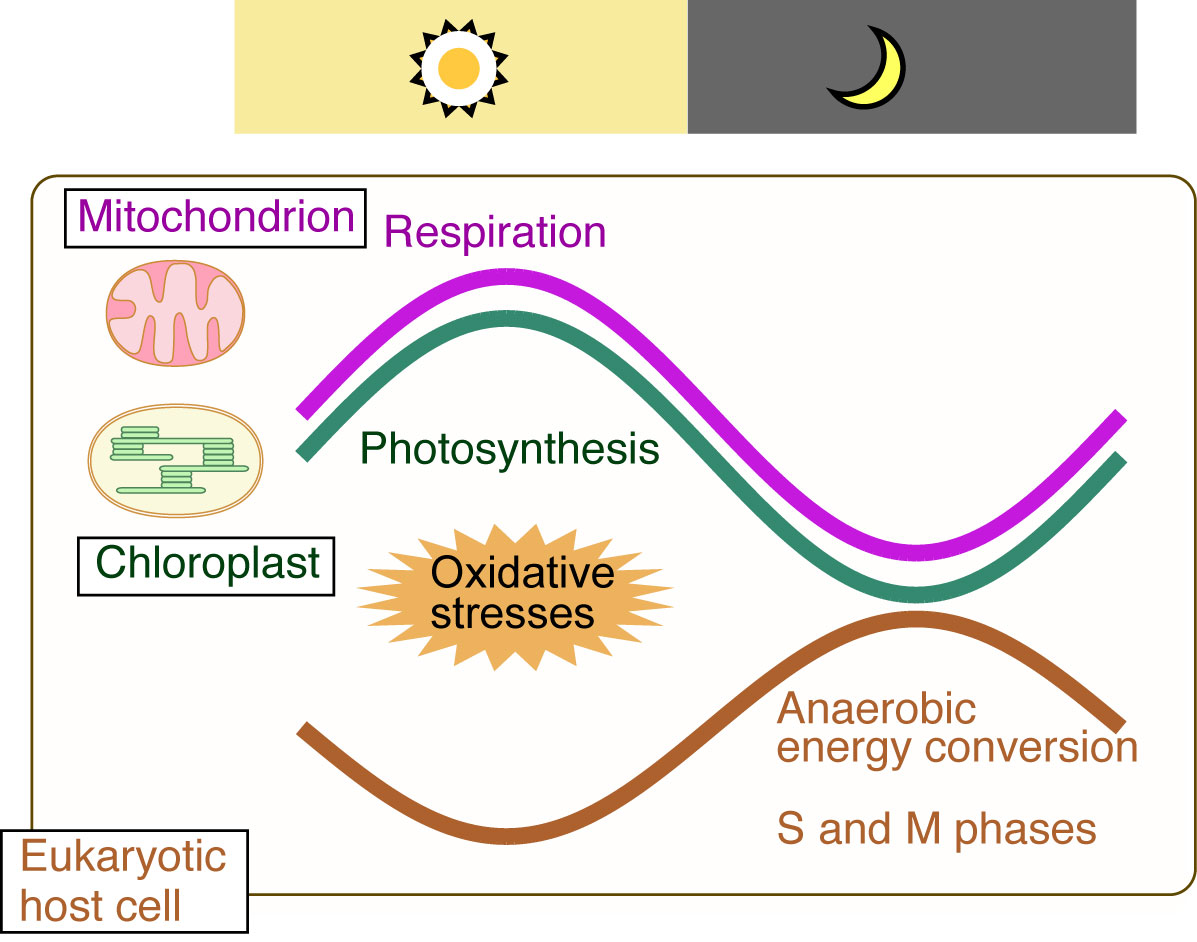
Figure2: The temporal separation of ROS-generating oxygenic energy metabolism by mitochondria and chloroplasts (endosymbiotic organelles) and nuclear DNA replication (eukaryotic host cell) ensures safe cell proliferation
Faculty member SHIMAMOTO at the Center for Frontier Research has been awarded tenure
NIG is proud to announce that an associate professor in the Center for Frontier Research has been awarded tenure as of July 1, 2019.
SHIMAMOTO, Yuta: Physics and Cell Biology Laboratory
Center for Frontier Research is an incubation center to simultaneously develop two elements: human resources and new research fields. Promising young scientists conduct research as principal investigator (tenure-track associate professor) to explore new frontiers in genetics and related areas, taking advantage of NIG’s research infrastructure and various support systems. Those who obtained tenure will establish new research divisions in NIG to lead the new fields that they contribute in creating.

- SHIMAMOTO, Yuta associate Professor















