Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Mechanism that regulates an efficient switch from cell elongation to cell division.
Division-site localization of RodZ is required for efficient Z ring formation in Escherichia coli
Yusuke Yoshii, Hironori Niki, Daisuke Shiomi
Molecular Microbiology 2019 DOI:10.1111/mmi.14217
Cell elongation and cell division are controlled by different complexes. The mechanisms that promotes a switch from elongation to division have been still unclear. It has been recently reported that a direct interaction between MreB actin, which regulates elongation, and FtsZ tubulin, which regulates division, promotes the switching at the division site. We have analyzed RodZ that interacts with MreB. In this paper, we found that RodZ also localized on the division site depending on FtsZ, and that MreB localized to the division site depending on RodZ. When RodZ is not localized to the division site, the formation of the division ring (Z ring) composed of FtsZ was delayed. The localization of RodZ to the division site promotes the division ring formation and efficient cell division. This work was supported by KAKENHI and NIG-JOINT A.
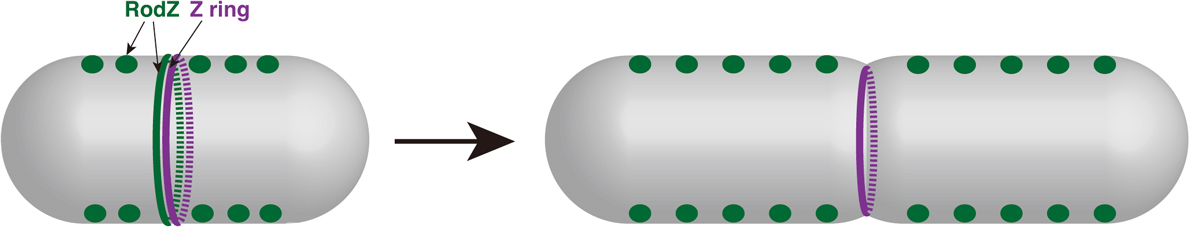
Figure: Division site localization of RodZ promotes an efficient formation of Z ring (i.e. switch from elongation to division).
Dynamic chromatin organization
Dynamic chromatin organization without the 30-nm fiber
Kazuhiro Maeshima, Satoru Ide and Michael Babokhov
Current Opinion in Cell Biology Volume 58, June 2019, Pages 95-104 DOI:10.1016/j.ceb.2019.02.003
Chromatin in eukaryotic cells is a negatively charged polymer composed of DNA, histones, and various associated proteins. Over the past ten years, our view of chromatin has shifted from a static regular structure to a dynamic and highly variable configuration. While the details are not fully understood yet, chromatin forms numerous compact domains that act as dynamic functional units of the genome in higher eukaryotes. By altering DNA accessibility, the dynamic nature of chromatin governs various genome functions including RNA transcription, DNA replication, and DNA repair/recombination. Based on new evidence coming from both genomics and imaging studies, we discuss the structural and dynamic aspects of chromatin and their biological relevance in the living cell.
This research was supported by JST CREST(JPMJCR15G2), JSPS Kakenhi (16H04746) and Takeda Science Foundation.
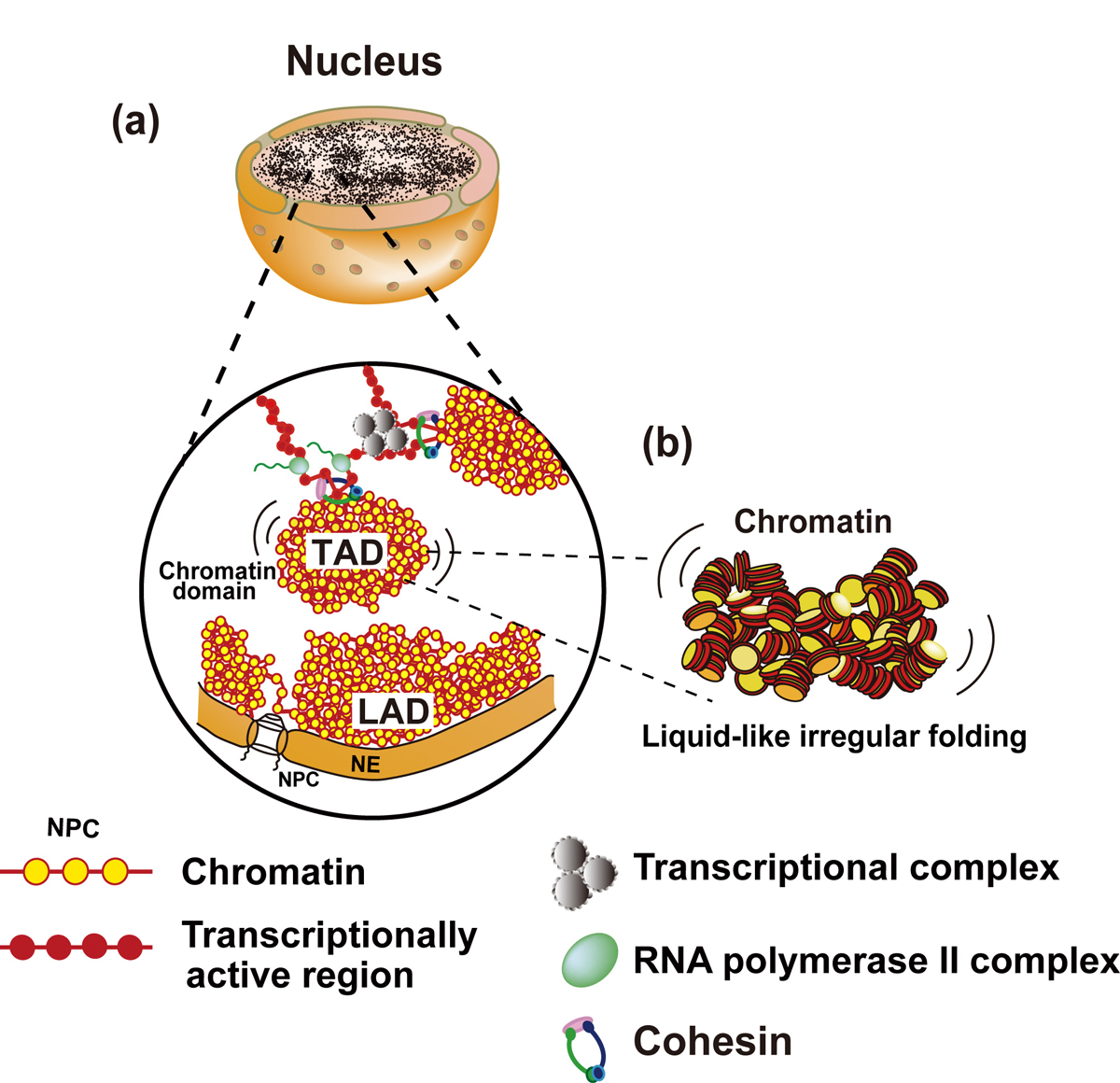
Figure: Interphase chromatin structure
(a and b) Chromatin consists of irregularly folded 10 nm fibers and forms numerous chromatin domains (e.g., topologically associating domains or contact/loop domains). The domains are formed by cohesin and nucleosome-nucleosome interactions. The compact domains could behave similar to a “liquid drop”. Binding of large transcriptional complexes (grey spheres) and RNA polymerase II (green spheres) might constrain movements of chromatin domains. Chromatin regions attached to nuclear envelop (NE) are called LADs (lamina-associated domains). NPC, nuclear pore complex; NE, nuclear envelop.
Transcription regulates dynamic movements of genomic DNA in living human cells
Press release
Single nucleosome imaging reveals loose genome chromatin networks via active RNA polymerase II
Ryosuke Nagashima, Kayo Hibino, S. S. Ashwin, Michael Babokhov, Shin Fujishiro, Ryosuke Imai, Tadasu Nozaki, Sachiko Tamura, Tomomi Tani, Hiroshi Kimura, Michael Shribak, Masato T.Kanemaki, Masaki Sasai, and Kazuhiro Maeshima
Journal of Cell Biology Published March 1, 2019 DOI:10.1083/jcb.201811090
Press release (In Japanese only)
The human body is composed of over forty trillion cells. Within each of these cells there is close to two meters of tightly packaged genomic DNA, the blueprint of life. Recently, there have been many advances in understanding how DNA is packaged and organized in the cell. In contrast, how DNA behaves mostly remains unknown.
In this recently published report, SOKENDAI graduate student Ryosuke Nagashima, assistant professor Kayo Hibino and professor Kazuhiro Maeshima of the National Institute of Genetics partnered with assistant professor S.S. Ashwin and professor Masaki Sasai of Nagoya University to study the movements of DNA in living cells using super resolution fluorescence microscopy (Figure 1A and B). Previously, it was commonly thought that the process of reading genomic information, known as transcription, would lead to more dynamic movements of DNA through the loosening of nucleosome packing. However, as Nagashima et al. report, transcription normally restricts DNA movement since the inhibition of transcription leads to increased DNA movements (Figure 1C). Furthermore, this work revealed that RNA polymerase II and other transcription-related factors form hubs to limit the motion of DNA (Figure 2). These results suggest that hubs may network the genome, restricting DNA movement to enable the efficient execution of transcription.
The results of this research have advanced our understanding as to how genetic information is acquired from DNA and may provide further clues concerning related disease states caused by abnormal changes to transcription.
This research is a result of a collaboration between the Genome Dynamics Laboratory of the National Institute of Genetics (Ryosuke Nagashima, Kayo Hibino, Michael Babokhov, Sachiko Tamura, Tadasu Nozaki, Ryosuke Imai, Kazuhiro Nagashima), the Nagoya University Department of Computational Science and Engineering (S.S. Ashwin, Shin Fujishiro, Masaki Sasai), the Cell Biology Center, Institute of Innovative Research, Tokyo Institute of Technology (Hiroshi Kimura), the Division of Molecular Cell Engineering of the National Institute of Genetics (Masato T. Kanemaki) and the Eugene Bell Center for Regenerative Biology and Tissue Engineering, Marine Biological Laboratory, Woods Hole (Michael Shribak, Tomomi Tani).
This research was supported by JST CREST(JPMJCR15G2), JSPS Kakenhi(16H04746), Takeda Science Foundation, RIKEN Pioneering Project, NIG-JOINT(2016-A2 (6)), SOKENDAI and National Institute of General Medical Sciences grant (R01-GM101701).
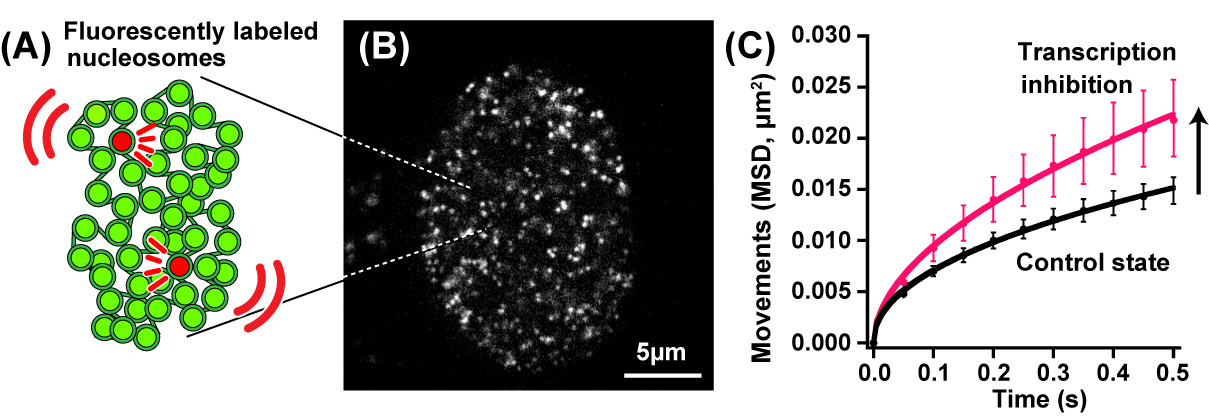
Fig.1. (A) A small fraction of nucleosomes was fluorescently labeled (red). (B) A single-nucleosomes image of a living human cell. (C) Genomic DNA motion, which is shown as mean square displacement, MSD), increased upon transcription inhibition.
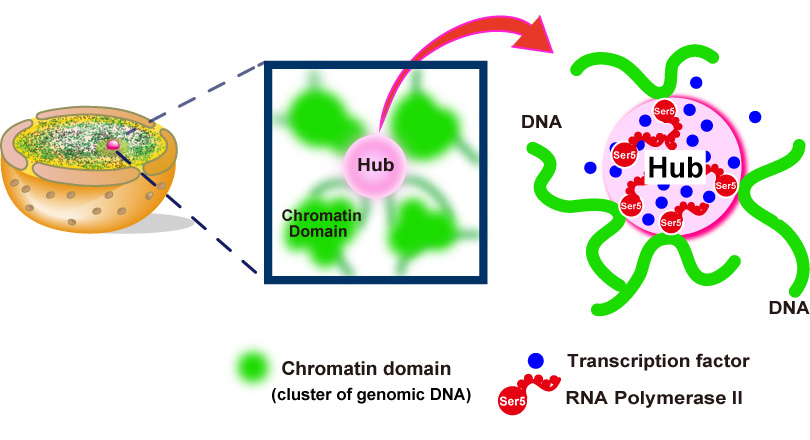
Fig.2. Hubs (pink spheres) of RNA polymerase II (red) and other components (blue spheres) of the gene transcription machinery constrain genomic DNA movements by connecting different genome regions together into an organized network.
Video1: Movie data (50 ms/frame) of single nucleosomes fluorescently labeled in a living human cell. Note that clear, well-separated dots and their movements were visualized.
Video2: Movie data (50 ms/frame) of single nucleosomes upon transcription inhibition. Note that greater movements of the dots are observed than those in Video1, suggesting the local genome dynamics increased upon transcription inhibition.















