Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Development of organs in living whole embryo/larval grafts in zebrafish
Model Fish Genomics Resource Laboratory / Sakai Group
Development and growth of organs in living whole embryo and larval grafts in zebrafish
Toshihiro Kawasaki, Akiteru Maeno, Toshihiko Shiroishi & Noriyoshi Sakai
Scientific REPORTS, 7: 16508 DOI:10.1038/s41598-017-16642-5
Age-related systemic environments influence neurogenesis and organ regeneration of heterochronic parabiotic partners; however, the difficulty of manipulating small embryos prevents the effects of aged systemic environments on primitive organs at the developmental stage from being analysed.
Here, we describe a novel transplantation system to support whole living embryos/larvae as grafts in immunodeficient zebrafish by the intrusion of host blood vessels into the grafts, allowing bodies similar to those of heterochronic parabiosis to be generated by subcutaneous grafting. Although grafted embryos/larvae formed most organs, the liver, testes and heart developed insufficiently or even occasionally disappeared. Removal of host germ cells stimulated testis development in grafted embryos. These results indicate that primitive testes are susceptible to the systemic environments that originated from the germ cells of aged hosts and imply that the primitive liver and heart are similar. This unique transplantation system will lead to new insights into the age-related systemic environments that are crucial for organogenesis in vertebrates.
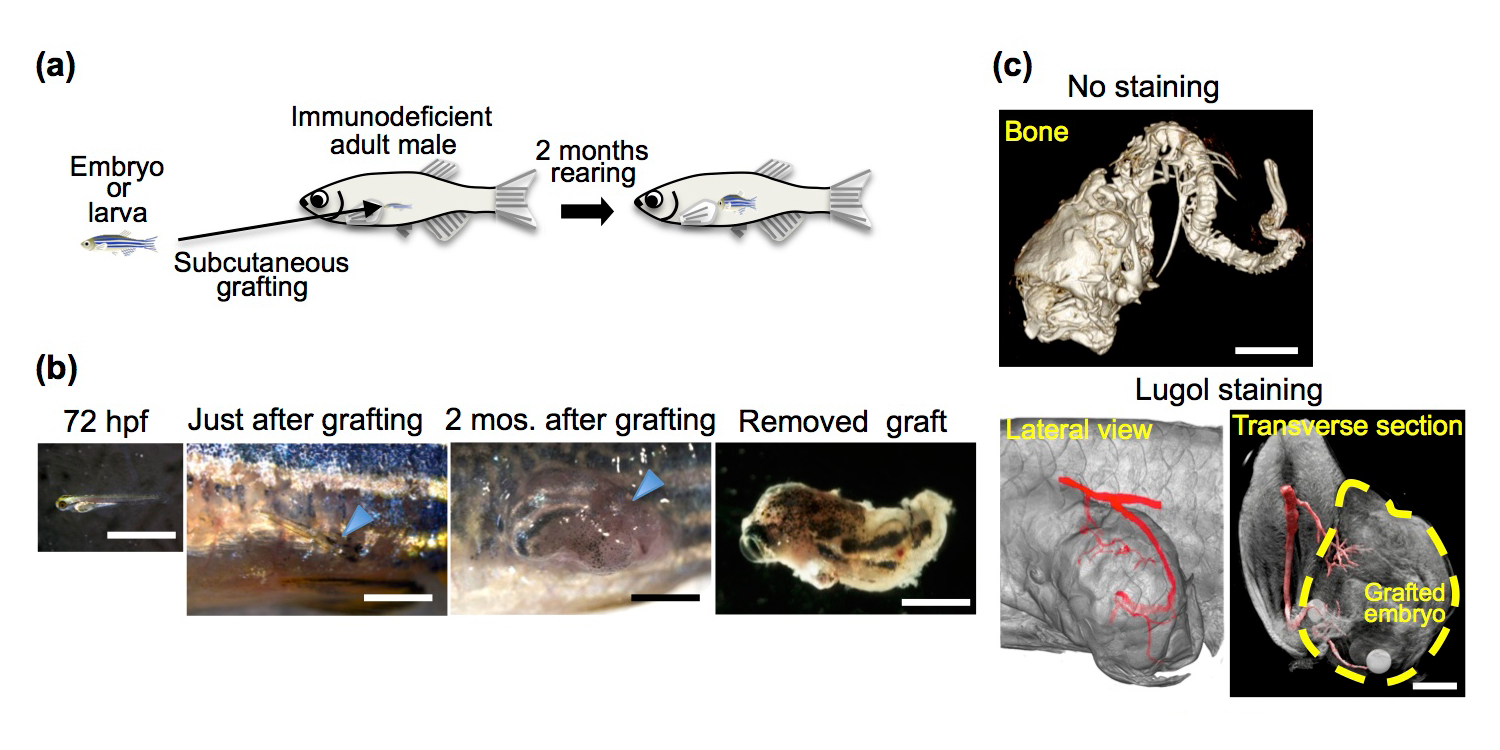
Development of grafted 72 hpf embryos in rag1 mutant hosts. (a) Schematic of whole-embryo transplantation. (b) Representative 72 hpf embryos and grafted embryos. (c) µ-CT imaging of the grafted embryo 2 months after transplantation. Cranial bones, vertebrae (No staining) and a vascular structure (Lugol staining) were observed in the grafted embryos.
You can see the several data of micro CT-scan which is one of the methods for this research below. NIG 3d Imaging Room (facebook)
3D Imaging Room in NIG(sketchfab)
An asymmetric attraction model for the diversity and robustness of cell arrangement in nematodes
Press release
An asymmetric attraction model for the diversity and robustness of cell arrangement in nematodes
Kazunori Yamamoto, Akatsuki Kimura
Development 2017 144: 4437-4449; DOI:10.1242/dev.154609
Pressrelease (In Japanese only)
During early embryogenesis in animals, cells are arranged into a species-specific pattern in a robust manner. Diverse cell arrangement patterns are observed, even among close relatives (Fig. A). In the present study, we evaluated the mechanisms by which the diversity and robustness of cell arrangements are achieved in developing embryos. We successfully reproduced various patterns of cell arrangements observed in various nematode species in Caenorhabditis elegans embryos by altering the eggshell shapes (Fig. B). The findings suggest that the observed diversity of cell arrangements can be explained by differences in the eggshell shape. Additionally, we found that the cell arrangement was robust against eggshell deformation. Computational modeling revealed that, in addition to repulsive forces, attractive forces are sufficient to achieve such robustness (Fig. C). The present model is also capable of simulating the effect of changing cell division orientation. Genetic perturbation experiments demonstrated that attractive forces derived from cell adhesion are necessary for the robustness. The proposed model accounts for both diversity and robustness of cell arrangements, and contributes to our understanding of how the diversity and robustness of cell arrangements are achieved in developing embryos.
This research article was published in the special issue of Development ‘On Growth and Form – 100 years on’, inspired by the seminal work of D’Arcy Thomson. See the authors’ comments:
Kazunori Yamamoto, the first author of the paper received Morishima Award for this research.
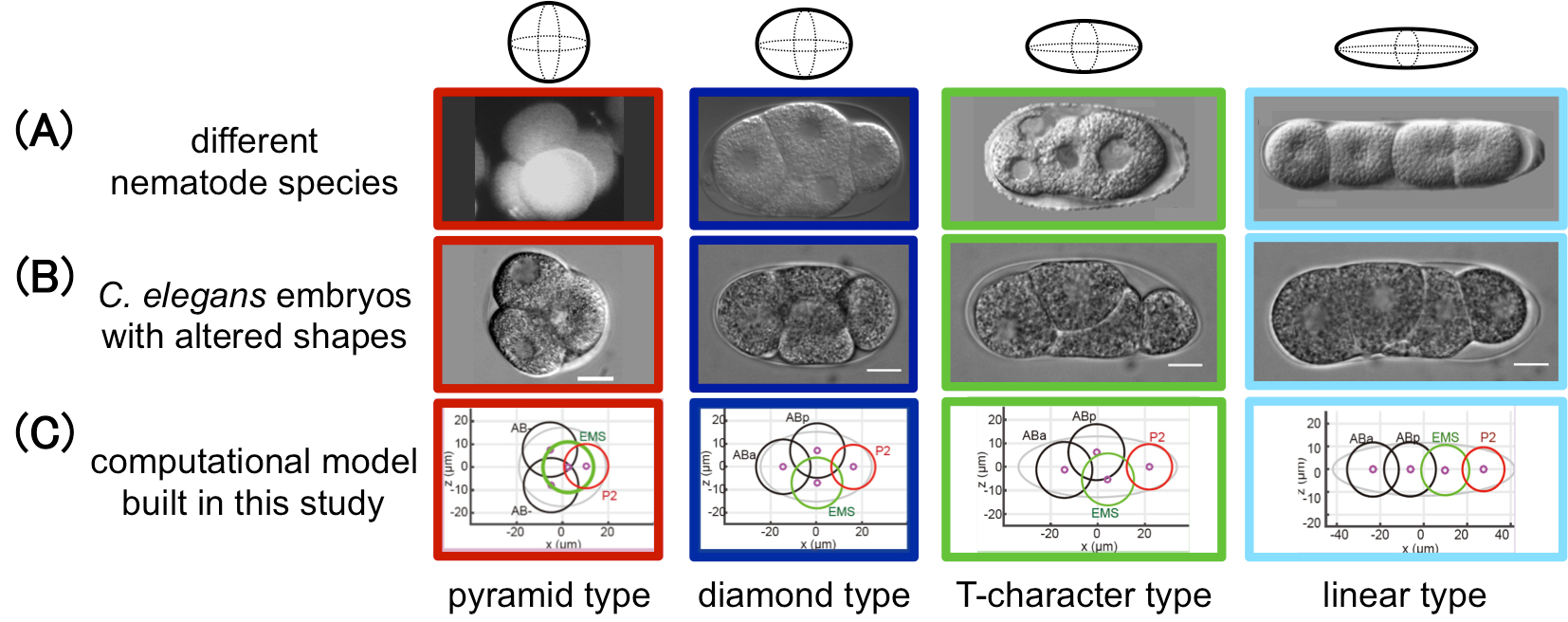
Figure: This study successfully reproduced the cell arrangement patterns of various nematode species (A) using C. elegans by altering the shape of the eggshell (B). A computational model was also built in this study that reproduces the diversity and robustness of the cell arrangement patters (C). In (A), the patterns of Enoplus brevis (red, image from Schulze and Shierenberg, Evodevo 2, 18, 2011), C. elegans (blue, this study), Cephalobus sp. (green, Goldstein, Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 1521-1531, 2001), and Belonolaimus sp. (light blue, Goldstein, 2001) are shown.
Source: Development 2017 144: 4437-4449
DOI: 10.1242/dev.154609
Conditional Degrons for Controlling Protein Expression at the Protein Level
Division of Molecular Cell Engineering / Kanemaki Group
Conditional Degrons for Controlling Protein Expression at the Protein Level
Toyoaki Natsume and Masato T. Kanemaki
Annual Review of Genetics, DOI:https://doi.org/10.1146/annurev-genet-120116-024656
It is a common genetic analysis in many organisms to see the phenotype after depletion of a protein of interest. Technologies such as siRNA and conditional gene knockout have been employed for this purpose. However, these do not deplete a protein of interest directly. It is, therefore, the depletion effect is indirect and it takes for a relatively long time to deplete a protein of interest, depending on the half-life of the protein. This sometime causes a problem because the phenotype can be complicated by a secondary effect that was initiated by a primary defect.
Recently, conditional degron technologies, by which a degron-fused protein can be induced for rapid degradation, are seizing the attention. Protein depletion by the degron technologies work directly at the protein level, so that it is possible to analyze the direct effect after rapid protein depletion. We developed the auxin-inducible degron technology by which a degron-fused protein can be controlled by a plant hormone auxin. Others used temperature, small chemicals, light, or the expression of another protein for the similar purpose. We wrote a review paper explaining the development history of conditional degrons and their characteristics.
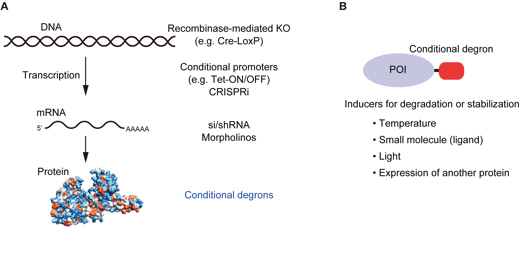
(A) Conditional technologies for controlling protein expression. From the level of gene to protein, various technologies have been developed. Conditional degrons work at the protein level. (B) Conditional degrons can be controlled by temperature, small chemicals, light, or the expression of another protein.
A novel mechanism that regulates decondensation of mitotic chromosomes
Microbial Genetics Laboratory / Niki Group
Release of condensin from mitotic chromosomes requires the Ran-GTP gradient in the reorganized nucleus.
Keita Aoki and Hironori Niki
Biology Open, November 15; 6(11):1614-1628, 2017 DOI:10.1242/bio.027193.
After mitosis, nuclear reorganization occurs together with decondensation of mitotic chromosomes and reformation of the nuclear envelope, thereby restoring the Ran-GTP gradient between nucleus and cytoplasm. The Ran-GTP gradient is dependent on Pim1/RCC1. Interestingly, a defect in Pim1/RCC1 in Schizosaccharomyces pombe causes post-mitotic condensation of chromatin, namely hyper-condensation, suggesting a relationship between the Ran-GTP gradient and chromosome decondensation. However, how Ran-GTP interacts with chromosome decondensation is unresolved. To examine this interaction, we used Schizosaccharomyces japonicus, which is known to undergo partial breakdown of the nuclear membrane during mitosis. We found that Pim1/RCC1 was localized on nuclear pores, but this localization failed in a temperature-sensitive mutant of Pim1/RCC1. The mutant cells exhibited hyper-condensed chromatin after mitosis due to prolonged association of condensin on the chromosomes. Conceivably, a condensin-dephosphorylation defect might cause hyper-condensed chromatin, since chromosomal localization of condensin is dependent on phosphorylation by cyclin-dependent kinase (CDK). Indeed, CDK-phospho-mimic mutation of condensin alone caused untimely condensin localization, resulting in hyper-condensed chromatin. Together, these results suggest that dephosphorylation of CDK sites of condensin might require the Ran-GTP gradient produced by nuclear pore-localized Pim1/RCC1.
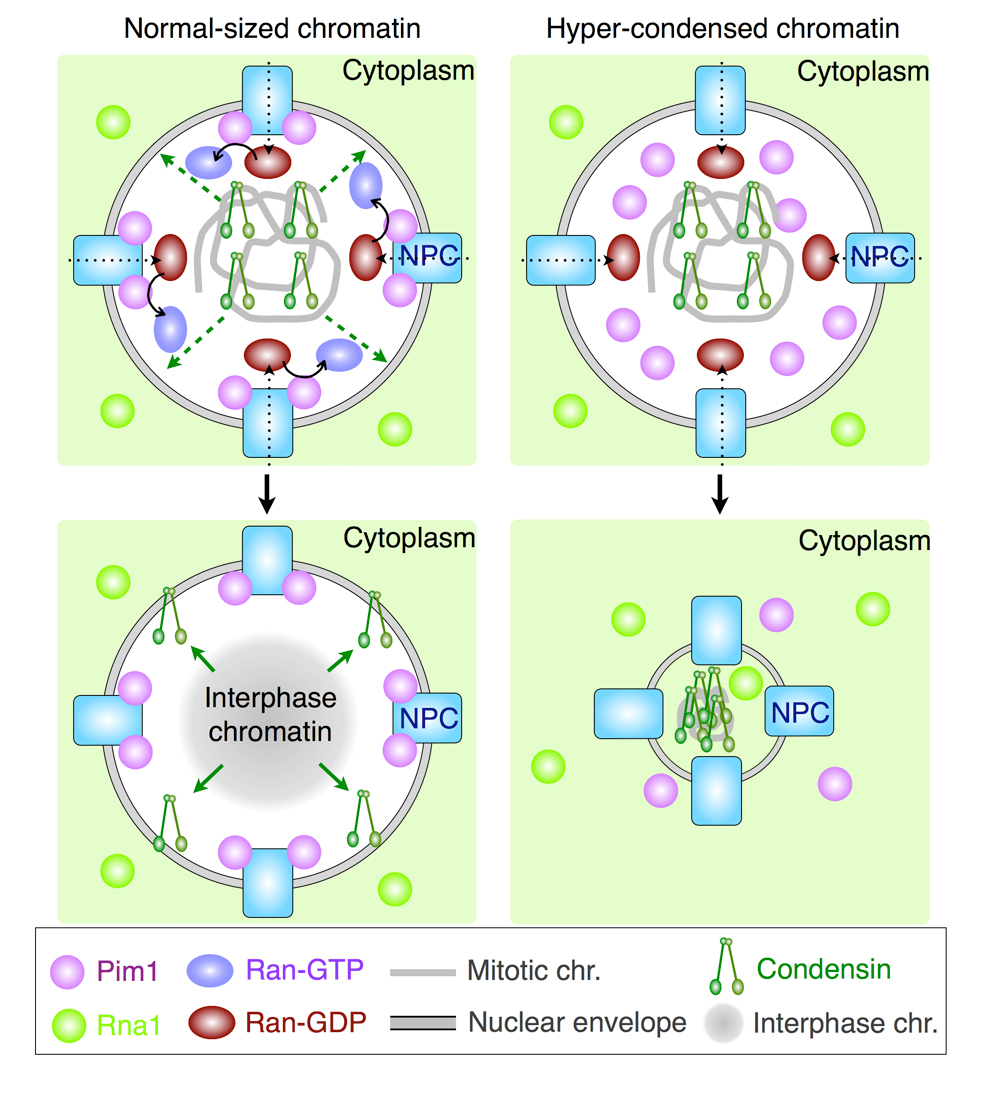
In a normal-sized nucleus (left), condensin is released from chromosomes in a Ran-GTP dependent manner. Therefore, a decondensed chromatin is formed in G1 phase. In a Pim1/RCC1-deficient nucleus, condensin is not released from chromosomes due to few Ran-GTP. Therefore, a hyper-condensed chromatin is formed in G1 phase.
A new function of rDNAs to compact bacterial chromosome
Microbial Genetics Laboratory / Niki Group
Multiple cis-acting rDNAs contribute to nucleoid separation and recruit the bacterial condensin, Smc-ScpAB
Koichi Yano, and Hironori Niki
Cell Reports, Vol. 21 Iss. 5, October 31, 2017 DOI:http://dx.doi.org/10.1016/j.celrep.2017.10.014
Condensins load onto DNA to organize chromosome. Smc-ScpAB clearly loads onto the parS sites bound by Spo0J, but other loading site(s) must operate independently of parS. In this study, we asked where and how Smc-ScpAB normally selects its loading site. Our results suggest that rDNA is also a loading site. A pull-down assay revealed that Smc-ScpAB preferentially loads onto rDNA in the wild-type cell and even in a Δspo0J mutant, but not in a Δsmc mutant. Moreover, we showed that deletion mutants of rDNAs cause a defect in nucleoid separation, and at least two rDNAs near oriC are essential for separation. Full-length rDNA including promoters is required for loading and nucleoid separation. A synthetic defect by deletions of both rDNA and spo0J resulted in more aberrant nucleoid separation. We propose that a single-stranded segment DNA that is exposed at highly transcribed rRNA operons would become a target for Smc-ScpAB loading.
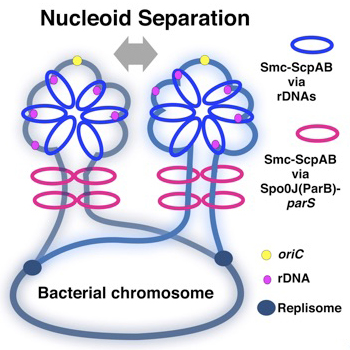
Schemes of compaction of newly replicated DNA strands. The yellow circle indicates oriC, black blue circles indicate replication forks, and magenta circles indicate rrn operons. The blue rings indicate Smc-ScpAB topologically loaded on rrn operons, and magenta rings represent Smc-ScpAB topologically loaded by ParB bound to parS as shown in Wang et al. (2017). In addition to separation of oriC, chromosome compaction would always be accompanied by proper chromosome segregation. The chromosomal region adjacent to oriC is packed by Smc in the WT so that the nucleoids are clearly separated.
Combination of multiple Shh enhancers controls tooth development in mouse
Mammalian Genetics Laboratory / Shiroishi Group
SHH signaling directed by two oral epithelium-specific enhancers controls tooth and oral development
Tomoko Sagai, Takanori Amano, Akiteru Maeno, Hiroshi Kiyonari, Hyejin Seo, Sung-Won Cho and Toshihiko Shiroishi
Scientific Reports, 7, Article number: 13004 (2017) DOI:10.1038/s41598-017-12532-y
Interaction between the epithelium and mesenchyme coordinates patterning and differentiation of oral cavity structures including teeth, palatal rugae and tongue papillae. SHH is one of the key signaling molecules for this interaction. Epithelial expression of Shh in the tooth buds and tongue papillae is regulated by at least two enhancers, MRCS1 and MFCS4. However, it is unclear how the two enhancers cooperate to regulate Shh. Here, we found that simultaneous deletion of MRCS1 and MFCS4 results in the formation of a supernumerary tooth in front of the first molar. Since deletion of either single enhancer barely affects tooth development, MRCS1 and MFCS4 evidently act in a redundant fashion. Binding motifs for WNT signaling mediators are shared by MRCS1 and MFCS4, and play a central role in regulating Shh expression, indicating that the two redundant enhancers additively exert their Shh regulation by responding to WNT signal input.
This study was carried out as a collaboration of Tomoko Sagai, Takanori Amano and Toshihiko Shiroishi of National Institute of Genetics, Hiroshi Kiyonari of RIKEN Center for Life Science Technologies, and Hyejin Seo and Sung-Won Cho of Yonsei University in Korea. This study was supported by JSPS KAKENHI 24247002.
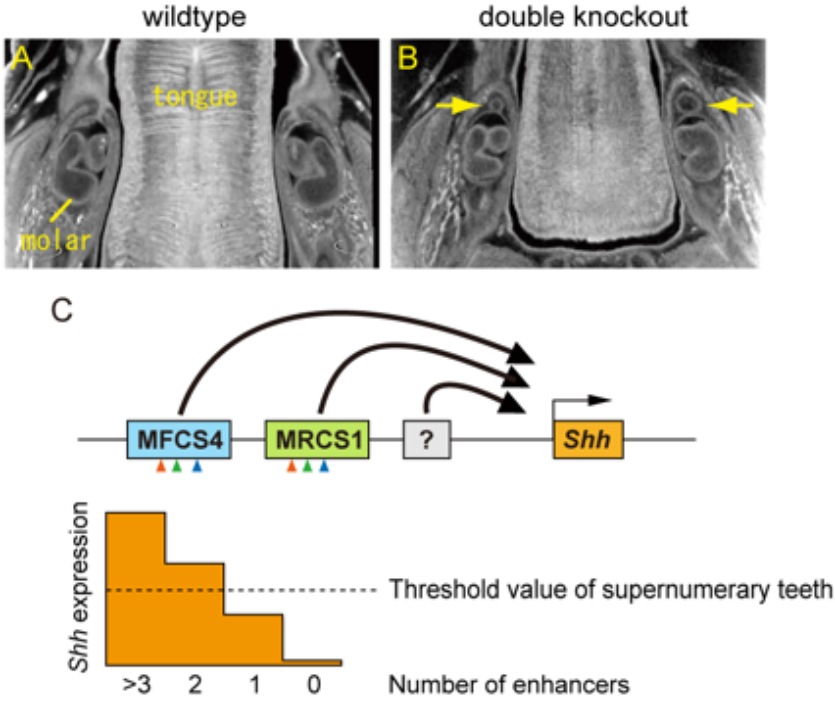
Supernumerary tooth formation in the mouse with simultaneous deletion (double knockout) of the MRCS1 and MFCS4 enhancers. (A and B) X-ray micro-CT images of transverse section of mandible in the wild type (A) and the mouse with the combinatorial deletion of MRCS1 and MFCS4 (B). Yellow arrow indicates supernumerary tooth. (C) A model of redundant action of the Shh enhancers. MRCS1 and MFCS4 share three common regulatory motifs that are located in the same order (arrowheads) and have redundant roles for the Shh expression in tooth buds. Expression levels of Shh are additively regulated by MRCS1, MFCS4, and probably unidentified enhancers. When reduction of Shh expression level falls below a threshold, supernumerary molars arise. MRCS1 and MFCS4 control different expression domains of Shh in the oropharyngeal tissues in addition to the tooth bud. Thus, the two redundant enhancers may cooperatively regulate the tooth development, while they have undergone sub-functionalization.















