PIGN prevents protein aggregation in the ER
Multicellular Organization Laboratory / Sawa Group
PIGN prevents protein aggregation in the endoplasmic reticulum independently of its function in the GPI synthesis
Shinji Ihara, Sohei Nakayama, Yoshiko Murakami, Emiko Suzuki, Masayo Asakawa, Taroh Kinoshita and Hitoshi Sawa
J Cell Sci 2017 130: 602-613; DOI:10.1242/jcs.196717
1. Protein aggregation is a common feature of many neurodegenerative diseases and often results from defective folding processes. We found that PIGN functions in protein quality control to prevent protein aggregation within the ER in C. elegans and in mammalian cells. Although PIGN was originally identified as an enzyme involved in glycosylphosphatidylinositol (GPI)-anchor biosynthesis, we show that the function of PIGN in protein quality control is independent of its enzymatic activities. In human, several mutations in PIGN have been shown to cause multiple congenital anomalies-hypotonia-seizures syndrome 1 (MCAHS1) that shows dysmorphic facial features and Fryns syndrome that cause lethality in the neonatal period. C. elegans with pign mutations created by CRISPR/Cas9 that correspond to MCAHS1 patients also cause protein aggregation in the ER, implying that the dysfunction of the PIGN non-canonical function might affect symptoms of MCAHS1 and potentially those of other diseases.
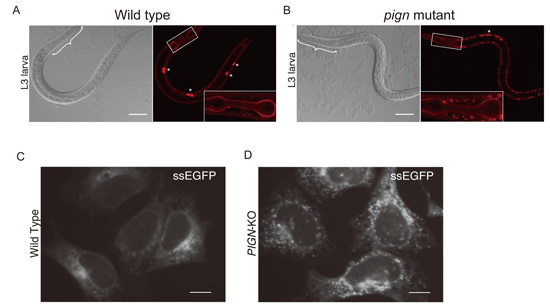
Accumulation of secretory proteins in C. elegans and mammalian cells with PIGN mutations.
(A) The localization of collagen-IV::mCherry (right) and differential interference contrast (DIC) images (left) of wild-type (A) and PIGN mutant (B) C. elegans.
The localization of secreted soluble EGFP (ssEGFP) in wild-type (C) and PIGN-knockout (D) HEK293 cells.















