Structural study of the homology domain of the eukaryotic DNA replication proteins Sld3/Treslin
Biomolecular Structure Laboratory • Shirakihara Group Division of Microbial Genetics • Araki Group
Crystal structure of the homology domain of the eukaryotic DNA replication proteins Sld3/Treslin
Hiroshi Itou, Sachiko Muramatsu, Yasuo Shirakihara, and Hiroyuki Araki Structure Published: August 7, 2014 DOI:10.1016/j.str.2014.07.001The initiation of eukaryotic chromosomal DNA replication requires formation of an active replicative helicase at the replication origins of chromosomal DNA. Yeast Sld3 and its metazoan counterpart Treslin are the hub proteins mediating protein associations critical for the helicase formation. In this study we show the crystal structure of the central domains of Sld3 that is conserved in Sld3/Treslin family proteins. The domain consists of two segments with 12 helices and is sufficient to bind to Cdc45, the essential helicase component. The structural model of the Sld3-Cdc45 complex that is crucial for formation of the active helicase was proposed.
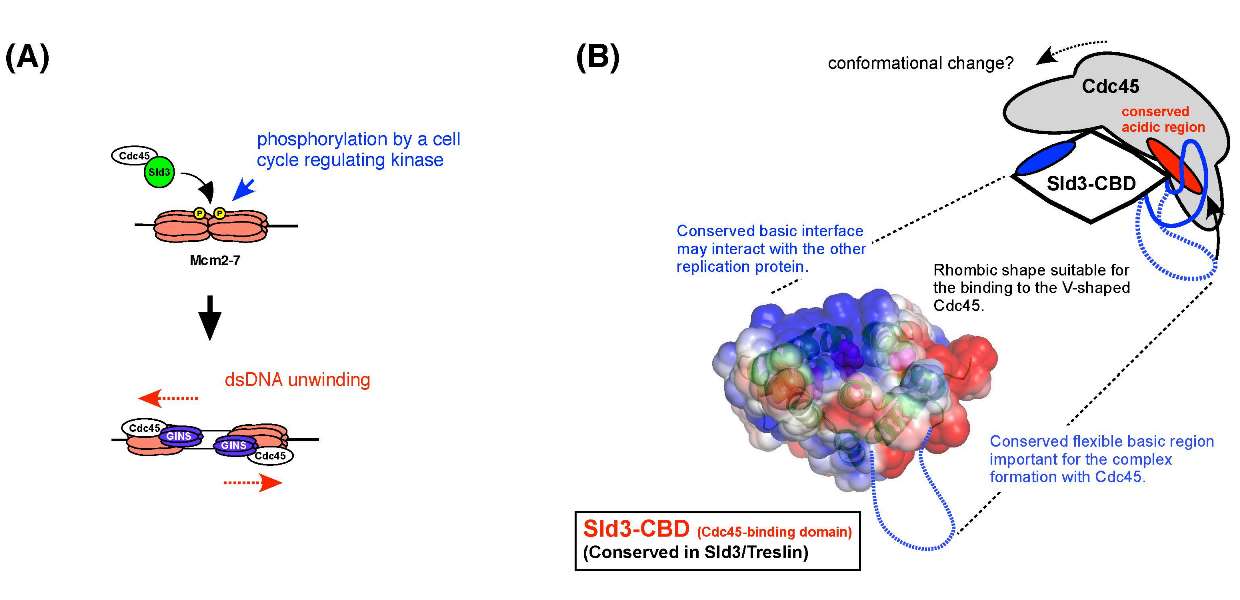
(A) Sld3 and Cdc45 form a complex that associates with origins in a mutually dependent manner,and its association to origins depends on the phosphorylation of the Mcm2-7 helicase core complex. Further protein interactions through Sld3 recruits GINS, another essential component of the active helicase. (B) Representation of the tertiary structure of the homology domain shared by Sld3 and Treslin. The proposed model of Sld3-Cdc45 complex is also shown.















