Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Ms. Neha Mishra (D3 Student SOKENDAI at Division of Evolutionary Genetics)received SOKENDAI President’s Award.

X chromosome-autosomes incompatibility accelerates speciation: reproductive isolation by misregulation of X-linked genes
Press release
Evolutionarily Diverged Regulation of X-chromosomal Genes as a Primal Event in Mouse Reproductive Isolation
Ayako Oka, Toyoyuki Takada, Hironori Fujisawa, and Toshihiko Shiroishi PLoS Genet Accepted manuscript online, 2014, doi/10.1371/journal.pgen.1004301Reproductive isolation is important for speciation, because it suppresses free genetic exchange between two diverged populations and accelerates the genetic divergence. Hybrid sterility (sterility in hybrid animals), one of the reproductive isolation phenomena, is possibly caused by deleterious interactions between diverged genetic factors brought by two distinct populations. A mouse inter-subspecific X chromosome substitution strain, B6-ChrXMSM, in which X chromosome of west European subspecies-derived C57BL/6J is replaced by counterpart of Japanese subspecies-derived MSM/Ms strain, shows reproductive isolation characterized by male-specific sterility due to disruption of meiotic entry in spermatogenesis (Oka et al, Genetics, 2004, 2007, 2010). In this study, we conducted comprehensive transcriptional profiling of the testicular cells of this strain by microarray. The results clearly revealed gross downregulation of gene expression in the substituted donor X chromosome. Such misregulation occurred prior to detectable spermatogenetic impairment, suggesting that it is a primal event in reproductive isolation. Furthermore, this misregulation subsequently resulted in perturbation of global transcriptional regulation of autosomal genes, probably by cascading deleterious effects. Remarkably, this transcriptional misregulation was substantially restored by introduction of chromosome 1 from the same donor strain as the X chromosome. This finding implies that one of regulatory genes acting in trans for X-linked target genes is located on chromosome 1. This study collectively suggests that regulatory incompatibility is a major cause of reproductive isolation in the X chromosome substitution strain.
This study was done as a research project “Life Systems” of Trans- disciplinary Research Integration Center, ROIS.
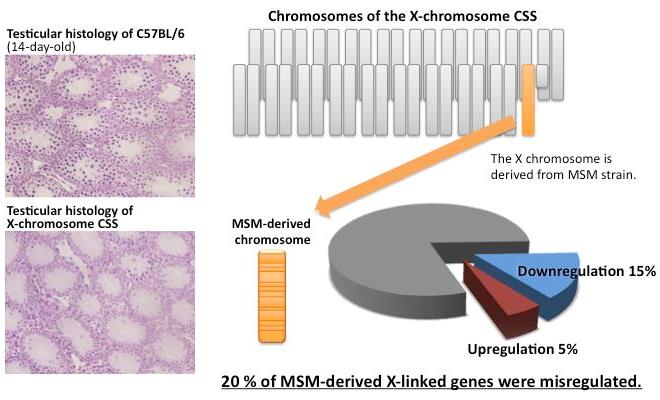
Figure 1. Misregulation of X-linked genes in the X-chromosome substitution strain (CSS). Meiotic spermatocytes were observed in testes of wild type (left upper panel), whereas they were rarely observed in testes of X-chromosome CSS (left bottom panel). Approximately 20% of expressed X-linked genes were misregulated in the X-chromosome CSS.
The inhibitory role of the medial prefrontal cortex on aggressive behavior in male mice
Press release
Control of Intermale Aggression by Medial Prefrontal Cortex Activation in the Mouse.
Aki Takahashi, Kazuki Nagayasu, Naoya Nishitani, Shuji Kaneko, Tsuyoshi Koide PLOS ONE April 16, 2014 doi:10.1371/journal.pone.0094657The adaptive nature of aggressive behavior in social animals accounts for its observation throughout the animal kingdom. However, given that aggressive behavior is no longer adaptive when it exceeds the species-typical level, mechanisms should curb excessive aggression to keep it within the adaptive range. The prefrontal cortex has been implicated in the inhibitory control of emotional outbursts, including aggression and violence. In this study, we used optogenetics to demonstrate that temporal activation of excitatory neurons in the medial prefrontal cortex (mPFC), but not the orbitofrontal cortex (OFC), inhibits inter-male aggression in mice. Activation of the mPFC suppresses aggressive bursts and reduces the intensity of aggressive behavior, but does not change the duration of the aggressive bursts. Our findings suggest that mPFC activity inhibits the initiation and execution, but not the termination, of aggressive behavior, and maintains such behavior within the adaptive range.
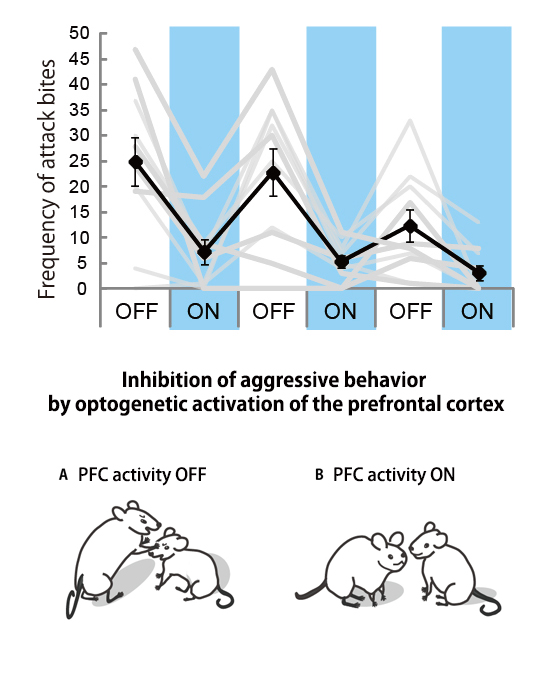
Optogenetic activation of the mPFC reduced the frequency of attack bites.
ON: light stimulation (blue light, 20 Hz, 5 mW), OFF: no light stimulation, gray lines: attack bites in each individual, black line: mean values.















