KANEMAKI, Masato D. Sc., Associate Professor

- Molecular Function Laboratory/ Kanemaki Group
- Present Post (since 2010)
Assistant Professor, Department of Biological Sciences, Graduate School of Science, and Faculty of Science Osaka University, Japan
Postdoctoral Fellow, Cancer Research UK
Ph.D., Chiba University, Japan
(born in 1974)
Hobbies: Cycling
Exploring animal cells using his original AID method
Associate Professor Masato KANEMAKI developed the epoch-making AID (auxin inducible degron) method, which degrades target proteins in cells within a short period of 15 to 30 minutes by making use of the characteristics of auxin, a plant hormone. At present, Dr. Kanemaki is trying to use this unique method to analyze the mechanism of proliferation of animal cells. What is the AID method, and what does it allow scientists to do in research? We talked with Dr. Kanemaki.
- AID, an epoch-making protein degradation technology
- Auxin, a plant hormone related to embryonic development, phototropism and gravitropism of plants, was discovered in the 1940s and is mentioned in high school biology textbooks. However, it was only in 2005 that the detailed reaction mechanism of auxin was reported. Based on this knowledge, Dr. Kanemaki developed the AID (auxin inducible degron) method, published a paper about it in December 2009, and obtained a patent for it.
- “To put it simply,” Dr. Kanemaki explains, “the AID method is used to extremely rapidly degrade a specific target protein in cells by adding the plant hormone called auxin to the cell culture medium. Examining the phenotype that is triggered by the depletion of the protein enables us to determine the function of the protein.”
- In plant cells, auxin combines the protein complex SCF ubiquitin ligase with the AUX/IAA proteins. As a result, AUX/IAAs are ubiquitinated and quickly degraded by the proteasome.
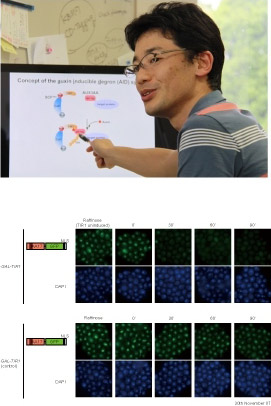 “Our starting idea for the AID method was hijacking this mechanism. We surmised that by attaching one of AUX/IAAs to a target protein like a tag it would be recognized only where there is auxin around and then destroyed by proteasome.”
“Our starting idea for the AID method was hijacking this mechanism. We surmised that by attaching one of AUX/IAAs to a target protein like a tag it would be recognized only where there is auxin around and then destroyed by proteasome.”- SCF ubiquitin ligase is found in all eukaryotic cells, but the TIR1 protein, which is a SCF subunit recognizing AUX/IAAs, is existing only in plants. Therefore, by introducing TIR1 into non-plant cells and fusing target proteins with AUX/IAA, they can be degraded when auxin is added.
- The laboratory tested this idea first with yeast cells. TIR1 was genetically cloned from a plant for expression, and AUX/IAA was attached to a target protein to degrade. When auxin was added to the yeast culture, the target protein was degraded in about 30 minutes. The group went on to test the technique with a variety of other cells, including those of a monkey, hamster, mouse, and human, and the method functioned well with all the cells.
- “A target protein almost completely disappears in 15 minutes or so. I don’t think there’s any other way that can remove proteins at such a high speed. There are RNAi, transcription suppression, and other ways to stop protein production by turning off transcription, but they sometimes require several to scores of hours. The AID method, on the other hand, requires no more than half an hour. Another advantage of this method was developed from plant, with auxin representing almost no toxicity to animal cells. So we don’t have to worry about secondary effects.”
- How will it be possible to handle animal cells as easily as yeast?
- Previously, Dr. Kanamaki researched the replication and maintenance mechanism of chromosomes using budding yeasts. He developed the AID method as he began to use animal cells.
- “The starting point of my idea was the question: ‘how can we handle cultured cells as easily as yeasts?’ Experiments using yeasts usually advance smoothly because there is a whole range of genetic technologies available. This is why over the last 30 to 40 years yeasts have greatly contributed to biological research. But what we scientists and others really want to know is the function of proteins in mouse and human cells. But there is no easy genetic technology applicable for the functional characterization of proteins in such cells. That’s why I thought better technologies should be made available.”
- To study the function of a protein in the cells, it is best to observe what happens to the cell when the protein is no longer there. With temperature-sensitive yeast mutants, for example, it is possible to study the function of a target protein by inactivating it by temporarily raising the culture temperature. This approach cannot be simply applied to animal cells because the temperature shift can affect the cells and sometimes even kill them. On the other hand, the AID method involves attaching a tag to a target protein in cultured cells and applying auxin to conduct an experiment. Cells do not die and are not exposed to any other impact than auxin.
 “When you have been working in one research area for a long while, it becomes difficult to free yourself from what has become the established standard in that area. Since I have moved from yeasts to cultured cells, I thought about creating something extremely convenient for myself that would allow me to handle cultured cells the way I used to treat yeasts. I think the many others simply didn’t have that kind of imagination that could be put into action.”
“When you have been working in one research area for a long while, it becomes difficult to free yourself from what has become the established standard in that area. Since I have moved from yeasts to cultured cells, I thought about creating something extremely convenient for myself that would allow me to handle cultured cells the way I used to treat yeasts. I think the many others simply didn’t have that kind of imagination that could be put into action.”- Science and technology as two wheels that work together
- “I believe that technology has given birth to new fields of science, and developments in these fields have in turn advanced technology. Without microscopes, we could not observe cells. In reality, continuous improvements in microscopy have allowed scientists to learn a lot about cells, and as we know more about cells, our sphere of interest has expanded. Science and technology grow hand in hand, like two wheels of a vehicle that move together.”
- Dr. Kanemaki’s laboratory intends to pursue research on two main pillars of technology and basic research into cultured cells. Regarding technology, they are exploring new ways of making use of the AID method.
- “The AID method is about degrading target proteins. Auxin puts Protein A and Protein B together. This means that other applications are possible: for example, if we devise a way to control degradation, Proteins A and B can come together but without disappearing. This is a little bit like cell engineering. If we perfect artificial convergence or separation of proteins under certain conditions, we can largely broaden the scope of experiments. So I’d like to do research in this direction as well.”
- Regarding cultured cells, the laboratory has several themes relating to the stability of chromosomes.
- “Cells have a mechanism to correct errors that occur during DNA duplication. How does this mechanism work? We know that when this doesn’t function correctly, it leads to cellular cancer, death, or aging. I wish to directly demonstrate this mechanism using mouse or human cells. If breakthrough discoveries were made thanks to the AID method, that would be great advertising for the technology, too. So I hope that we will produce successful results on our own.”
- Products of interdisciplinary interaction
 Dr. Kanemaki first had the idea for the AID method while listening to a conversation in the botanical research laboratory adjacent to his.
Dr. Kanemaki first had the idea for the AID method while listening to a conversation in the botanical research laboratory adjacent to his.- “The professor in the laboratory told me about this interesting degradation system in plants. That was the very beginning. I find plants fascinating! Imagine this: a botanist thinking only about plants on one side, and an animal specialist thinking only animals on the other. One day they meet, compare notes, and find something interesting, or potentially valuable, in the other’s research project. I believe that interdisciplinary interaction is important.”
- Dr. Kanemaki has received offers of joint research from other laboratories of the NIG. “Some nearby laboratories want to use the AID method. So we’re preparing to work together while continuing discussions. I am experiencing the joy of a creator of technology. It’s great that the AID method can be used by other laboratories and even researchers in different fields who find it useful. I’m very grateful for this opportunity to be able to contribute to the research community in this way.”
- What discoveries and what products will result from those new collaborative projects? There is much to expect from joint research in the future.
- (Interviewed by Yoshiko Tamura 2011)















