Mcm8-9: a bridge among anti-cancer agent sensitivity, DNA recombination repair, and Fanconi anemia disease

Kanemaki Group’s basic research centering on the twin pillars of technological development and cell biology aims at elucidating the mechanisms of chromosome replication, repair and recombination.
Kanemaki Group’s recent joint research with scientists from Kyoto University, Osaka University and the NIG, namely Associate Professor Kazuki Horikawa (currently Designated Professor at the University of Tokushima) and Prof. Tatsuo Fukagawa, resulted in the discovery of a protein complex related to DNA interstrand crosslink repair. The research results were published in Molecular Cell.
The protein complex is composed of Mcm8 and Mcm9, already identified as members of the same family of components of the DNA replication machine. Little was known about their functions in animal cells.
In only one and a half years after his arrival at the NIG in October 2010, Dr. Kanemaki succeeded in having his research results published. In the interview that follows, we asked the scientists about the research and its high and low moments.
Research possible only at the NIG, “Research Paradise”
What led you to focus your attention on Mcm8 and Mcm9 in the first place?

Kanemaki (K): Originally, we were looking into the mechanism of DNA replication. The most important part of DNA replication is the process during which DNA is divided into two. This process is catalyzed by the ring-shaped replicative DNA helicase, composed of Mcm2-7 proteins.
Mcm 8-9 were initially identified as proteins similar to Mcm2-7, but their function had not been very clear. We wanted to clarify their function. That was our starting point.
If your research concerns a factor which seems important but whose function is unclear, aren’t you running into intense competition?
K: Yes, there’re many scientists who think like us. In fact, we have overseas rivals with regard to our recently published paper. I think their paper is also published in the same issue of Molecular Cell. We met those scientists at an overseas conference and realized that we were doing the same research.
That encounter was useful in that we were able to exchange information, but it added a little pressure to our research.
You used chicken DT40 cells in your research this time, not yeasts as you used to do before. You established a new experimental system and put together research results in a paper in the first one and a half years since your arrival at the NIG. I suppose that you put in enormous efforts.
K: I was greatly helped by several factors. Firstly, my research experience with yeasts, because the same method of analysis can be used even when you work with different organisms. Secondly, Prof. Fukagawa, who knows DT40 cells so well; it was as if we worked together while I learned experiment techniques from him. Thirdly, the NIG’s research environment complemented my lab, which was not very well equipped because we were doing experiments that we had never done before. The NIG had all sets of equipment for common use that was available at any moment when it was necessary. Finally, being surrounded by scientists who were doing research in neighboring fields was great. We learned a great deal by communicating with them.
We have one postgraduate student in our group, and we tell him that the NIG may not be the best place if you want to work in a company after graduation, but this is a Paradise for those who want to be a scientist by doing nothing but research, totally immersed in research.
Excited at the prospect of Mcm8-9 as a comprehensive explicatory factor
What is the highlight of your paper?
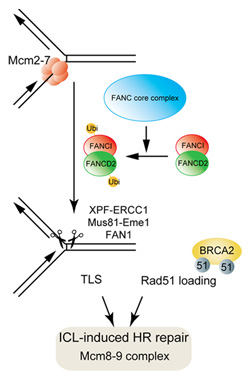
K: What is most important about this research is that cells deficient in Mcm8 and Mcm9 are hypersensitive to specific drugs such as cisplatin and mitomycin C, which are DNA interstrand crosslink (ICL) agents.
This pattern of sensitivity is very similar to that of cells found in patients of Fanconi anemia (FA), hereditary disease characterized by hypersensitivity to DNA ICLs.
Fifteen genes responsible for FA have already been identified, and all of them are known to be related to DNA ICL repair. We can’t say for sure since we don’t do clinical research, but we believe that it is possible that Mcm8 and Mcm9 are also causative genes of FA.
When was the most exciting moment during the research?
K: That was when I began to see the possibility that our discovery could be used to comprehensively explain all the previous research findings. Most of the papers that had been published about Mcm8 and Mcm9 stated that they were involved in replication, but some contained unusual reports about, for example, Mcm8-deficient flies that have abnormal meiosis and prematurely menopausal women with mutated Mcm8. I believe that our discovery that Mcm8-9 is related to chromosome recombination repair may be able to comprehensively explain all those findings.
DNA recombination is related to a variety of phenomena of life such as DNA repair, meiosis and immunity, but they are all studied independently. I was very excited at the prospect of using Mcm8-9 as a comprehensive explicatory factor for all the previous findings.
Nishimura (N): There are three major moments. One was when we succeeded in producing knockout cells. That was supposed to be impossible because knocking out genes related to replication usually ends up killing cells. Since we had assumed that the function of Mcm8-9 was replication, it was quite a surprise when we obtained data that indicated another possibility.
Another big moment was when we discovered that cells deficient of Mcm8-9 was sensitive only to specific agents such as cisplatin and mitomycin C. The third moment was when we saw where Mcm8-9 was located in the cell.
Dr. Kanemaki said that if the protein complex was related to repair its location in specific regions in the nucleus should become visible, but I was skeptical. In the experiment, foci of Mcm8-9 were visible only when we damaged the cells. We were then convinced that it was definitely related to repair.
Genetics to comprehensively understand phenomena of chromosomes in higher animals
What are you planning to do next?
K: Our primary and ultimate research goal is to perfect clear genetic analysis of human cells.
We have always been hoping to apply the knowledge we have gained from research using yeasts to research on higher eukaryotic organisms. We also want to do genetically clear-cut research using cells that are totally deficient in target genes, as we have done with budding yeasts.
Two years ago, when we began experimenting, chicken DT40 cells were the only cultured cells of which we could knockout or modify target genes, except ES cells, which are extremely difficult to culture. So we used DT40 cells in our research. Still, our true wish is to experiment with human cells.
As preparatory steps toward realizing this wish, we have introduced some new technologies that enable us to neatly modify target genes in human cells. We are approaching the stage where we can produce cells deficient in target genes, instead of degrading them as with siRNA.
We also possess the AID (auxin inducible degron) system, capable of gene expression and degradation in specific timeframes. Usually, we end up with dead cells if we attempt to produce cells deficient in genes related to replication and recombination, which interest us most. But with the AID system coupled with genetic modification, we have come to the stage where we can freely control protein expression in human cells.
In many laboratories, DNA replication is studied as an independent phenomenon. However, from the perspective of chromosome, it probably shouldn’t be viewed separately. I think, for example, if a problem occurs to DNA during replication, repair could occur, or recombination may be used as a solution.
I believe that replication, repair and recombination occur in a collaborative manner. So I wish to understand the other phenomena involving chromosomes from a broad perspective, without excessively focusing on the core process of replication alone.
Kanemaki Group’s recent discovery will lead to new knowledge that would be essential to developing low-dose anti-cancer treatments, elucidating the mechanism of development of FA, and understanding the mechanisms of DNA replication, repair and recombination that are fundamentals of life.
Let us hope that further achievements will be made by Kanemaki Group, as its members attempt to unravel the mysteries of chromosomes through basic research founded on technological development and cell culture.
(Aug. 09, 2013)
Mcm8 and Mcm9 Form a Complex that Functions in Homologous Recombination Repair Induced by DNA Interstrand Crosslinks
Kohei Nishimura, Masamichi Ishiai, Kazuki Horikawa, Tatsuo Fukagawa, Minoru Takata, Haruhiko Takisawa, and Masato T. Kanemaki. Molecular Cell, Published online: 05 July 2012 DOI: 10.1016/j.molcel.2012.05.047
Back














