Hydrodynamics explains intra-cellular flows, just like bath water!

| Akatsuki KIMURA, D. Sc., Associate Professor, Cell Architecture Laboratory, Center for Frontier Research,National Institute of Genetics/ SOKENDAI. |
| Ritsuya NIWAYAMA, Ph.D Student, Department of Genetics, SOKENDAI. (Graduate University for Advanced Studies) |
Mr. Niwayama, what led you to research into intracellular flows?
One major reason is that I find dynamic flows inside the cells, which you can observe using a microscope, extremely fascinating. As an undergraduate at the University of Tokyo, I studied Physics and Mathematics until my third year and in the fourth year took up Molecular Biology for my graduation project. Through my undergraduate courses, I learned of Dr. Kimura, who had graduated from the same high school as me, Sapporo Minami, in Hokkaido. I arrived at the NIG to do postgraduate studies at SOKENDAI in April 2007, hoping to acquire a solid ability to conduct research.
I spent the first three months at the NIG defining my research theme. I decided to analyze intra-cellular transport. Generally, active transport of cargos along the cytoskeleton driven by motor proteins was then attracting the research community’s attention. Instead, I had come up with the idea of viewing intra-cellular transport as an overall cytoplasmic flow.
In the cells, there are proteins and RNAs that must be at their designated places to function properly, and they are transported by motor proteins and firmly fixed by specific proteins. There are also many other proteins and RNAs that drift inside the cells, and they are believed to be somewhat influenced by intra-cellular flows. It was vaguely believed that Hydrodynamics could explain the movements of the latter group of materials, but no scientifically solid analysis or explanation had not been done.
Could you explain the “overall cytoplasmic flow” a little more specifically?
It is like a gently flowing liquid. We conducted our research with Caenorhabditis elegans several minutes after fertilization. At this stage, the motor protein called myosin, which exists only along the cortex (surface) of the cell, causes a mono-directional flow near the cortex, whereas in the inner part a flow in the opposite direction is witnessed. It was believed that this was explained by the same principle as that of bath water flows, that is, a flow in one direction occurring near the edge of the bathtub results in another flow in the opposite direction in the inner part. We wanted to verify this. It was not very clear if the simple principle of Hydrodynamics could be applicable to cells, which are quite densely packed with proteins and other molecules, as well as various fibrous substances. So we wanted to examine actual flows and clarify.
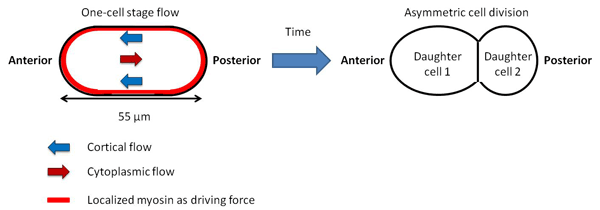
Why would the “overall cytoplasmic flow” be necessary in the cell of C. elegans immediately after fertilization?
We believe that it is necessary for asymmetrical cell division. Multicellular organisms start from a single cell, a fertilized egg, which undergoes repeated divisions and differentiations. For this process to occur, a cell must divide into two cells each with different properties. This is asymmetrical cell division, which is found in bone marrow stem cells, for example. Asymmetrical cell division requires polarity, that is, uneven distribution of materials, meaning specific proteins or RNA being at or around specific locations in the cells. If all materials were evenly distributed in the cells, the division of a cell could only result in two completely identical cells.
There are several mechanisms in which cytoplasmic polarity occurs, and we believe that the “overall cytoplasmic flow” is one of them. Since asymmetrical division and differentiation begin in C. elegans immediately after fertilization, we believe that the overall cytoplasmic flow is used for polarization for the initial asymmetrical division.
What experimentation and analysis did you carry out?
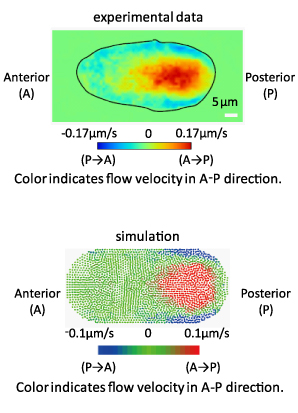
First of all, we made microscopic observations of intra-cellular flows immediately after fertilization and analyzed the data in detail using the particle image velocimetry (PIV) program in collaboration with Dr. Kyosuke Shinohara of Osaka University. We were able to obtain accurate numerical data that confirm that a cortical flow occurs in one direction and the opposite flow in the inner part and that the inner flow, which is slow at first, accelerates as it approaches the center of the cell. Next, we computer-simulated one-directional cortical flows to observe resultant inner flows, using the moving particle semi-implicit (MPS) method, which enables us to analyze flows inside pliant structures such as cells and blood vessels. As a result, we obtained almost exactly the same flows as we did in the experiment.
We also confirmed using RNAi that both cortical and inner flows cease when myosin expression is restrained. In other words, we pursued our research while directly verifying that intra-cellular streams are driven by myosin movements.
You succeeded in explaining the “overall cytoplasmic flow” with hydrodynamics, then?
Yes, you can say that. Our hydrodynamic simulation was the first to clearly explain intra-cellular flows, to which the principle of fluid dynamics used to be applied vaguely, with so solid scientific grounds. Our experiment using C. elegans can serve as the theoretical basis for research about intra-cellular flows in other organisms, as well as research about flows in blood vessels and lymph vessels.
What does the future hold now?
I would like to do research combining Experimental Biology and IT techniques. At present, I am trying to further refine the methodology used in the intra-cellular transport research project so that it may be used to analyze cell flows in blood streams and organisms in the embryonic stage. I am also interested in research that leads to generating some useful substances, such as bio-ethanol, in specified cells by integrating Biology with Engineering.
My last question is addressed to Dr. Kimura. How do you view Mr. Niwayama’s research?
In my laboratory, all the students carry out research according to their interest. This is how I have conducted my research. Mr. Niwayama has come to the NIG (SOKENDAI) to do research that combines Biology with Physics or Mathematics; I hope to support him as much as I can so that he can do what interests him most.
In the research project that we have discussed today, focus was placed on measurements and simulations, without consideration to how intra-cellular flows immediately following fertilization contribute to asymmetrical division and cell differentiation. While there are already quite a few Physics-inspired studies in Biology, Mr. Niwayama’s research is novel and unique in that the MPS method was applied to cells, and I believe that this is one of the reasons the research is highly appreciated. Moreover, by demonstrating that Hydrodynamics is applicable to research into intra-cellular flows, he laid the foundations for a range of future research themes. I think there will be various ripple effects from this research. I expect Mr. Niwayama to do his best so that he can realize his dreams.
(Interviewed by Naoko Nishimura on Jun. 22, 2011)
The hydrodynamic property of the cytoplasm is sufficient to mediate cytoplasmic streaming in the Caenorhabiditis elegans embryo.
Ritsuya Niwayama, Kyosuke Shinohara, Akatsuki Kimura. Proceedings of the National Academy of Sciences of the United States of America (PNAS),108 (27), 2011. DOI: 10.1073/pnas.1101853108
Back














