Retina-derived signals control pace of neurogenesis in visual brain areas but not circuit assembly
Kawakami Group / Laboratory of Molecular and Developmental Biology
Retina-derived signals control pace of neurogenesis in visual brain areas but not circuit assembly
Sherman, S., Arnold-Ammer, I., Schneider, M.W., Kawakami, K., and Baier, H.
Nature Communications (2023) 14, 6020 DOI:10.1038/s41467-023-40749-1
Brain development is orchestrated by both innate and experience-dependent mechanisms, but their relative contributions are difficult to disentangle. Here we asked if and how central visual areas are altered in a vertebrate brain depleted of any and all signals from retinal ganglion cells throughout development. We transcriptionally profiled neurons in pretectum, thalamus and other retinorecipient areas of larval zebrafish and searched for changes in lakritz mutants that lack all retinal connections. Although individual genes are dysregulated, the complete set of 77 neuronal types develops in apparently normal proportions, at normal locations, and along normal differentiation trajectories. Strikingly, the cell-cycle exits of proliferating progenitors in these areas are delayed, and a greater fraction of early postmitotic precursors remain uncommitted or are diverted to a pre-glial fate. Optogenetic stimulation targeting groups of neurons normally involved in processing visual information evokes behaviors indistinguishable from wildtype. In conclusion, we show that signals emitted by retinal axons influence the pace of neuro- genesis in visual brain areas, but do not detectably affect the specification or wiring of downstream neurons.
This study was conducted as collaboration with Baier lab at Max Planck Institute.
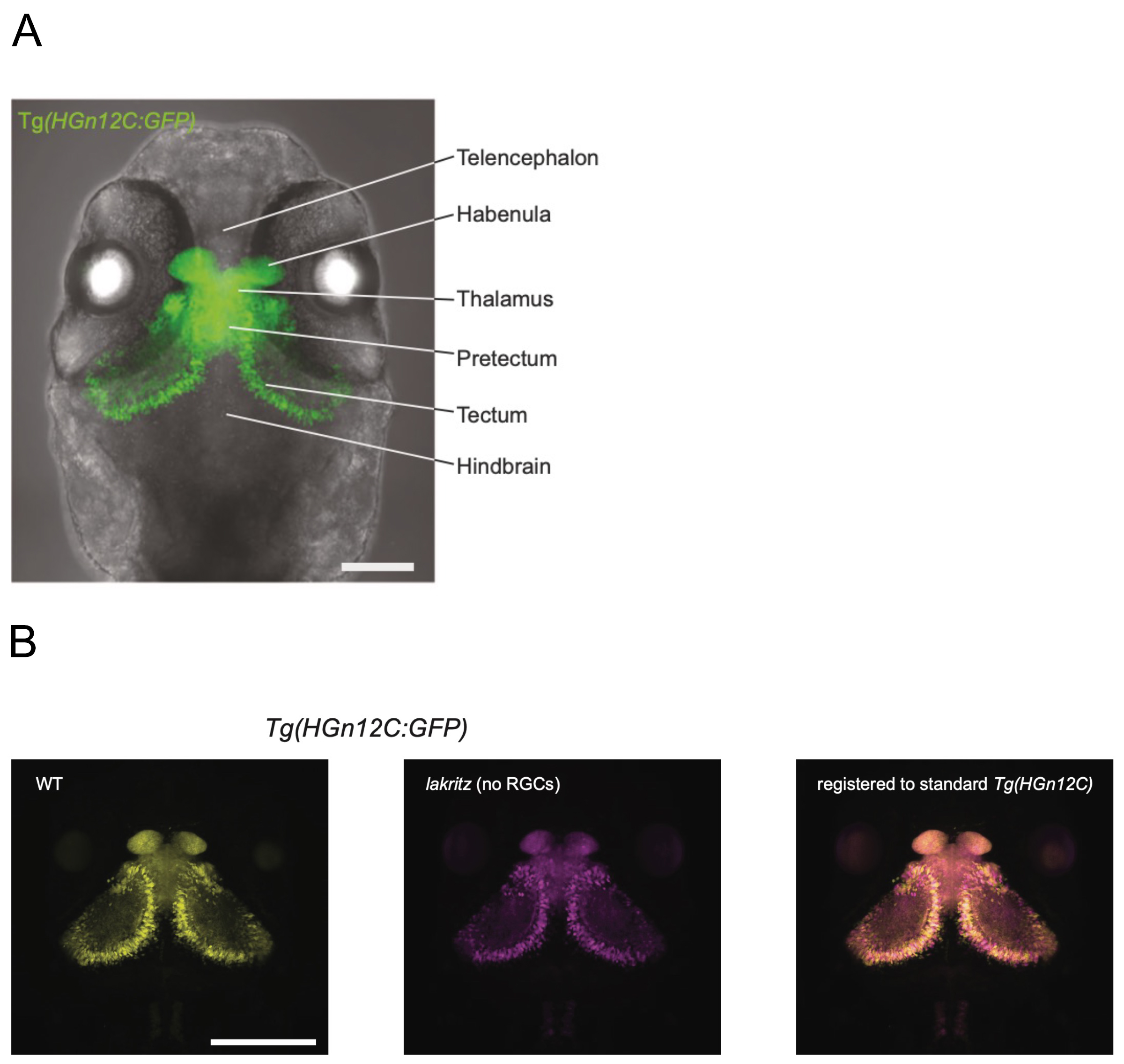
Figure: (A)Transgenic zebrafish (HGn12C) expressing GFP in neurons of retinorecipient areas.
(B)The lakritz mutation was introduced into HGn12C (center), and transcriptional profiling analysis of GFP-positive neurons was conducted.















