Illuminating the role of synapsis in subtelomeric recombination
Sycp1 Is Not Required for Subtelomeric DNA Double-Strand Breaks but Is Required for Homologous Alignment in Zebrafish Spermatocytes
Yukiko Imai, Kenji Saito, Kazumasa Takemoto, Fabien Velilla, Toshihiro Kawasaki, Kei-ichiro Ishiguro and Noriyoshi Sakai
Front Cell Dev Biol 9, 664377 (2021) DOI:10.3389/fcell.2021.664377
Meiosis is a specialized cell division, by which haploid gametes are produced from diploid cells for sexual reproduction. In meiosis, recombination mediates reciprocal exchanges of genetic contents between homologous chromosomes. In human and zebrafish males, meiotic recombination preferentially occurs near telomeres, but the mechanism underlaying the subtelomeric bias remains unknown.
In this study, we investigated roles of physical connection between homologous chromosomes (synapsis) in subtelomeric recombination by using zebrafish. We found that recombination begins near telomeres and homologous chromosomes pair locally, in synapsis-defective zebrafish. However, in the mutant zebrafish, a substantial proportion of pairing was lost in later stages, and no spermatozoa was generated. These observations revealed that synapsis is not essential for initiation of recombination at subtelomeres but is required for its completion.
Since the subtelomeric bias of recombination is also observed in human, new insights into zebrafish meiosis will facilitate understanding human infertility caused by meiotic defects.
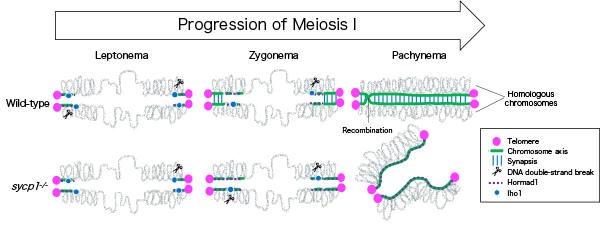
Figure: In wild-type zebrafish males, meiotic recombination begins with DNA double-strand breaks near telomeres. Synapsis is also initiated near telomeres at zygonema, and homologous chromosomes are paired up at pachynema. In sycp1 mutant males, DNA double-strand breaks and local and transient homologous pairing occur near telomeres.















