Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Identification of a cell population functioning during repair processes of notochord injury
Wilms Tumor 1b defines a wound-specific sheath cell subpopulation associated with notochord repair
Lopez-Baez, J.C., Simpson, D.J., Forero, L.L., Zeng, Z., Brunsdon, H., Salzano, A., Brombin, A., Wyatt, C., Rybski, W., Huitema, L.FA., Dale, R.M., Kawakami, K., Englert, C., Chandra, T., Schulte-Merker, S., Hastie, N.D., and Patton, E.E.
eLife 2018;7:e30657 DOI:10.7554/eLife.30657
Regenerative therapy for degenerative spine disorders requires the identification of cells that can slow down and possibly reverse degenerative processes. Here, we identify an unanticipated wound-specific notochord sheath cell subpopulation that expresses Wilms Tumor (WT) 1b following injury in zebrafish. We show that localized damage leads to Wt1b expression in sheath cells, and that wt1b+cells migrate into the wound to form a stopper-like structure, likely to maintain structural integrity. Wt1b+sheath cells are distinct in expressing cartilage and vacuolar genes, and in repressing a Wt1b-p53 transcriptional programme. At the wound, wt1b+and entpd5+ cells constitute separate, tightly-associated subpopulations. Surprisingly, wt1b expression at the site of injury is maintained even into adult stages in developing vertebrae, which form in an untypical manner via a cartilage intermediate. Given that notochord cells are retained in adult intervertebral discs, the identification of novel subpopulations may have important implications for regenerative spine disorder treatments.
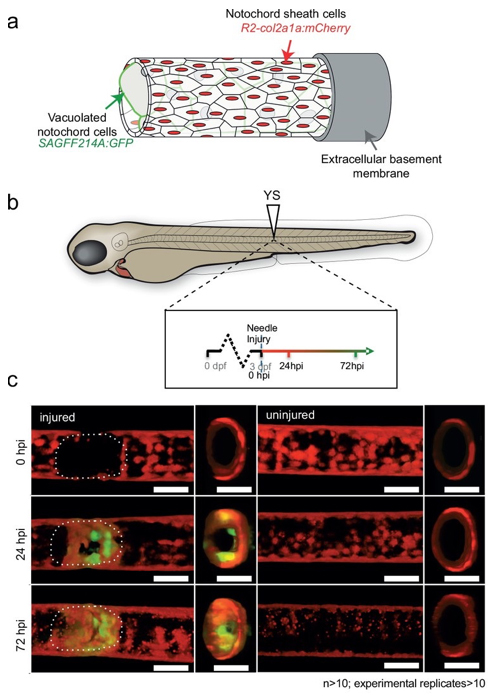
Figure: In transgenic fish expressing GFP in the notochord vacuolated cells, GFP-expressing cells appeared 24-72 hours after injury.
A mechanism how intestine tumors affects liver functions
A novel zebrafish intestinal tumor model reveals a role for cyp7a1-dependent tumor-liver crosstalk in tumor’s adverse effects on host
Sora Enya, Koichi Kawakami, Yutaka Suzuki, Shinpei Kawaoka
Disease Models & Mechanisms (2018) DOI:10.1242/dmm.032383
The nature of host organs and genes that underlie tumor-induced physiological disruption on host has been poorly understood. Here, we establish a novel zebrafish intestinal tumor model, and find that hepatic cyp7a1, the rate-limiting factor for synthesizing bile acids (bile alcohol (BA) in zebrafish) is important for such a phenomenon. We created a transgenic zebrafish line, in which an oncogenic form of kras (krasG12D) was expressed in the posterior intestine by the Gal4-UAS method, and found that the intestinal tumor was formed. The intestinal tumor then caused detrimental effects on host, including liver inflammation, hepatomegaly, growth defects, and organismal death. Whole-organismal level gene expression analysis and metabolite measurements revealed that the intestinal tumor reduced total BA levels possibly via altered expression of hepatic cyp7a1. We demonstrated overexpression of cyp7a1 in the liver restored the BA synthesis and ameliorated tumor-induced liver inflammation. Thus, we discovered a previously unknown role of cyp7a1 as the host gene that links the intestinal tumor to the hepatic cholesterol-BA metabolism and liver inflammation. Our model provides an important basis to investigate host genes responsible for tumor-induced phenotypes and to uncover mechanisms underlying how tumors adversely affect host organisms.
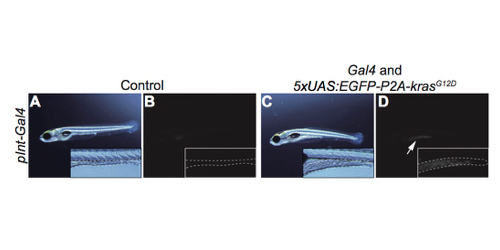
Figure: A transgenic zebrafish model bearing the intestine tumor created by the Gal4-UAS method. A, B; control. C, D: transgenic fish. B, D: fluorescent images. Dotted lines indicate the shape and size of the intestine. An arrow indicates EGFP expression.
Development of NGS data analysis platform, Maser
Maser: one-stop platform for NGS big data from analysis to visualization
Sonoko Kinjo, Norikazu Monma, Sadahiko Misu, Norikazu Kitamura, Junichi Imoto, Kazutoshi Yoshitake, Takashi Gojobori, Kazuho Ikeo
Database (2018) Vol. 2018: article ID bay027; DOI:10.1093/database/bay027
A major challenge in analyzing the data from high-throughput next-generation sequencing (NGS) is how to handle the huge amounts of data and variety of NGS tools and visualize the resultant outputs. To address these issues, we developed a cloud-based data analysis platform, Maser (Management and Analysis System for Enormous Reads), and an original genome browser, Genome Explorer (GE).
Maser realized a more user-friendly analysis platform especially for beginners by improving graphical display and providing the selected standard pipelines that work with built-in genome browser. All the analyses executed on Maser are recorded in the analysis history, helping users to trace and repeat the analyses. The entire process of analysis and its histories can be shared with collaborators or opened to the public. In conclusion, our system is useful for managing, analyzing, and visualizing NGS data and achieves traceability, reproducibility, and transparency of NGS analysis.
Maser is currently supported by Japan Agency for Medical Research and Development (AMED).
Figure 1: Maser tutorial and user registration page
Figure 2: Maser’s Top Page (Only registered user can access)
RinkaiHackathon: Lectures and practical training on seawater metagenome, June 10th-13th
RinkaiHackathon 2018
We set up a research group called “Rinkai Hack” with a few volunteers to promote research education and human resources exchanges beyond the boundaries of informatics and animal/ plant research, with marine stations as the primary places of activity. The name Rinkai Hackathon is derived from “Rinkai (coastal, waterfront, or marine), Hack, marathon”.
We widely invite for participants of the event of this year, “RinkaiHackathon 2018”.We will hold a symposium on June 10and hands-on, three-day course June 11-13.The venue of the symposium is Fukuju Kaikan in Fukuyama Castle, and the venue of the hands-on course is the Mukai-Shima marine station of Hiroshima University (Mukaijima, Onomichi, Hiroshima Prefecture).In addition to lectures by the committee members, several invited speakers are scheduled. For details, please see the event page.
https://sites.google.com/view/rinkaihack/
This year we are planning lectures and practical training on environmental DNA / metagenome and will provide experience-based learnings from DNA extraction from seawater to DNA sequence analysis. Let’s learn the actual latest situation of the marine metagenomic analyses which is often taking up as a scientific topic recently. At the same time, we will set a discussion time in which participants discuss what of classical knowledge and system of biology does not change depending on the progress of technology.
As of this year, we will change the primary language during the event to English, because we want to encourage domestic and international students to participate and also have opportunities to exchange outside the university. At the same time, we also encourage not only Japanese students who are confident in English but also students who are not as confident. If you cannot communicate well in English, the organizing staff will support you. (The committee members are also not very good at English. But we are doing it, somehow!! Que Sera Sera.)
For details of the event, please visit the website.
https://sites.google.com/view/rinkaihack/registration
Registration is accepted from the registration form on the website.
https://sites.google.com/view/rinkaihack/coming-events/rinkai-hackathon-2018
Any question related to the event is also accepted from the website.
https://sites.google.com/view/rinkaihack/coming-events/rinkai-hackathon-2018/moredetailsrinkaihackathon2018?authuser=0
Intended participants: graduate students and postdoctoral fellows.
Application deadline: May 11, 2018
* Since the practical training facility and training contents, we have a capacity of 20 people. In case of a large number of applications, we will conduct a selection.* There is a possibility of some change in the Hands-on schedule and the speaker’s schedule.
Confirmed Guest lecturers:
Shinsuke Kawagucci (JAMSTEC) at June 10 (Sun)Romain Blanc-Mathieu at June 10 (Sun)
Satoshi Yamamoto (Kyoto University) at June 11 (Mon)
Yusuke Uchiyama (Kobe University): oceanic dynamics at June 12 (Tue)
James Reimer (University of the Ryukyus) Schedule TBD
Satoshi Hiraoka (JAMSTEC) June 10 (Sun)
Yosuke Nishimura (Univ. of Tokyo) June 10 (Sun)
Rinkaihackathon2018 committee members
Masaaki Yoshida (Shimane University)
Takeshi Kawashima (National Institute of Genetics)
Mayuko Hamada (Okayama University)
Norio Miyamoto (JAMSTEC)
Yoshiaki Morino (University of Tsukuba)
Davin Setiamarga (NIT, Wakayama College)
Akihito Omori (Niigata Univ.)
Kunifumi Tagawa (Hiroshima University)
Co-hosted by
Hiroshima University
DDBJ: DNA Data Bank of Japan
JSBi (Japanese Society for Bioinformatics)
Supported by
ROIS Mirai Toushi FS
Endorsed by
The chair of the directors meeting for the Japanese National Marine and Inland Water Stations
Ingenious mechanism to ensure robust Polycomb silencing
![]()
Division of Neurogenetics
Mbf1 ensures Polycomb silencing by protecting E(z) mRNA from degradation by Pacman
Kenichi Nishioka, Xian-Feng Wang, Hitomi Miyazaki, Hidenobu Soejima, Susumu Hirose
Development 2018 145:dev162461 DOI:10.1242/dev.162461
Pressrelease (In Japanese only)
Under stress conditions, the coactivator Multiprotein-bridging factor 1 (Mbf1) translocates from the cytoplasm into the nucleus to induce stress-response genes. However, its role in the cytoplasm, where it is mainly located, has remained elusive. Here, we show that Drosophila Mbf1 associates with E(z) mRNA and protects it from degradation by the exoribonuclease Pacman, thereby ensuring Polycomb silencing. This mechanism would also allow flexibility in Polycomb silencing, as Mbf1 protein expression declines upon differentiation.
In addition to E(z) mRNA, Mbf1 binds to mRNAs of various stress-response genes. Therefore, Mbf1 appears to contribute to various types of stress defense as both a nuclear coactivator and as a cytoplasmic mRNA-stabilizing protein. It is intriguing that Mbf1 contributes to the same biological function through different subcellular localisation.
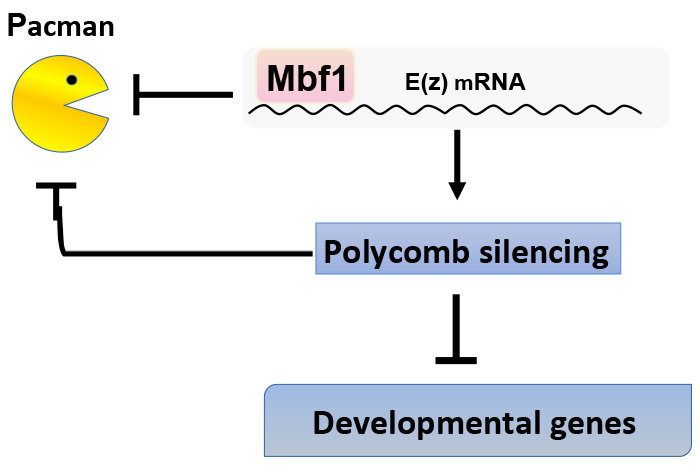
Mbf1 binds to E(z) mRNA and protects it from degradation by Pacman. Polycomb silencing represses expression of the pacman gene. Therefore, Mbf1 ensures robust Polycomb silencing.















