RING finger protein 121 facilitates the degradation and membrane localization of voltage-gated sodium channels.
Motor Neural Circuit Laboratory • Hirata Group
RING finger protein 121 facilitates the degradation and membrane localization of voltage-gated sodium channels
Ogino, K., Low, S. E., Yamada, K., Saint-Amant, L., Zhou, W., Muto, A., Asakawa, K., Nakai, J., Kawakami, K., Kuwada, J. Y., and Hirata, H.PNAS, 112: 2859-2864. DOI: 10.1073/pnas.1414002112
Voltage-gated sodium channels (NaV) are known to form clusters at the membranes of excitable cells; however, what governs their transport is largely unknown. We found that the endoplasmic reticulum (ER) and cis-Golgi associated ubiquitin ligase really interesting new gene (RING) finger protein 121 (RNF121) mediates the degradation and membrane localization of NaV. This apparent quality control of NaV ensures the transport of properly folded channels to the membranes of excitable cells. To our knowledge, this is the first pathologically relevant identification of a voltage-gated ion channel as a substrate for ER-associated protein degradation, whose degradation is governed by an ER- and Golgi-associated E3-ubiquitin ligase.
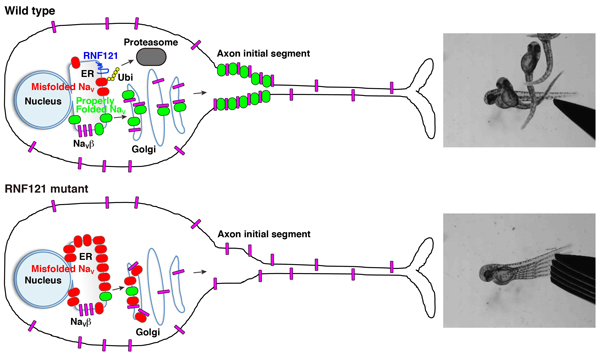
A wild-type neuron wherein RNF121 mediates ubiquitination of misfolded NaV channels marking them for proteasome-mediated degradation. Properly folded NaV channels (green) associate with NaVβ subunits (magenta) in the Golgi apparatus and are transported to the axon initial segment. Of note, some NaVβ subunits are transported to the membrane independent of NaV channels. An RNF121-deficient neuron wherein misfolded NaV channels (red) accumulates in the ER and cis-Golgi compartments, which, over time, depletes NaVβ subunits, preventing them from forming complexes with properly folded NaV channels, causing an impairment of NaV transport.















