Development of a New Method for Synthesis of Tandem Hairpin Pyrrole−Imidazole Polyamide Probes Targeting Human Telomeres
Biological Macromolecules Laboratory (Maeshima Group)
Development of a New Method for Synthesis of Tandem Hairpin Pyrrole–Imidazole Polyamide Probes Targeting Human Telomeres
Kawamoto, Y., Bando, T. *, Kamada, F., Li, Y., Hashiya, K., Maeshima, K. *, and Sugiyama, H. *
*co-corresponding authors
Journal of the American Chemical Society (JACS) October 1, 2013 DOI:10.1021/ja406737n
Pyrrole–imidazole (PI) polyamides bind to the minor groove of DNA in a sequence-specific manner without causing denaturation of DNA. To visualize telomeres specifically, tandem hairpin PI polyamides conjugated with a fluorescent dye have been synthesized, but the study of telomeres using these PI polyamides has not been reported because of difficulties synthesizing these tandem hairpin PI polyamides. To synthesize tandem hairpin polyamides more easily, we have developed new PI polyamide fragments and have used them as units in Fmoc solid-phase peptide synthesis. Using this new method, we synthesized four fluorescent polyamide probes for the human telomeric repeat TTAGGG. The polyamides synthesized using the new method successfully targeted to human and mouse telomeres under a physiological condition and allow easier labeling of telomeres in the cells while maintaining the telomere structure. Using the fluorescent polyamides, we demonstrated that the telomere length at a single telomere level is related to the abundance of TRF1 protein, a shelterin complex component in the telomere.
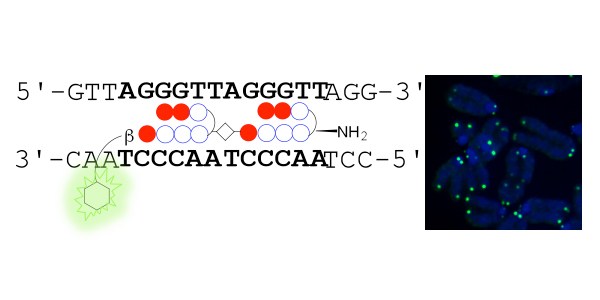
Left, schematic representation showing that TH59 compound with a fluorescent dye binds to the human telomere sequence (TTAGGG repeat). Right, the telomere regions of chromosome ends are labeled with TH59 (green). DNA stain (blue).















