The mechanism of synchronous pollen formation in an anther

Ken-ichi NONOMURA, D. Ag., Associate Professor,
Experimental Farm, National Institute of Genetics/ SOKENDAI.
What first prompted you to take interest in the meiosis of germ cells?
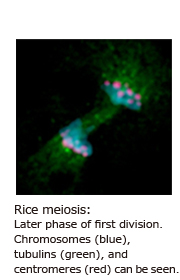
When I was an undergraduate student, an older lab member let me take a microscopic look at the meiosis of rice chromosomes. I found it very beautiful, and the mechanism that pairs homologous chromosomes during early meiosis intrigued me. So that was the beginning. Meiosis is characterized by a single round of premeiotic DNA replication followed by two rounds of chromosome segregation without DNA replication. During this process, the number of chromosomes carrying genetic information reduced by half the parents’. All organisms conducting sexual reproduction carry on a pair of homologous chromosomes from both of their parents. Prior to the first division of meiosis, the chromosome find their homologous partner and get matched up. This phenomenon called “homologous chromosome pairing” allows genes on the two parents’ sides to mix, producing spores and gametes with new genetic combinations that are in turn passed on to the child.
I used to think I wanted to develop rice varieties with practical utility when I was working in a university faculty engaged in plant breeding. I didn’t pursue that path after all, but I have always carried out research using rice. Since I arrived at the NIG 14 years ago, I started full-scale research into meiosis and pre-meiotic processes. I select phenotypes showing the absence of pollen or seeds from among rice mutants and analyze their function mainly with approaches of forward genetics.
What gene did you identify in your study, and what did you discover about meiosis?
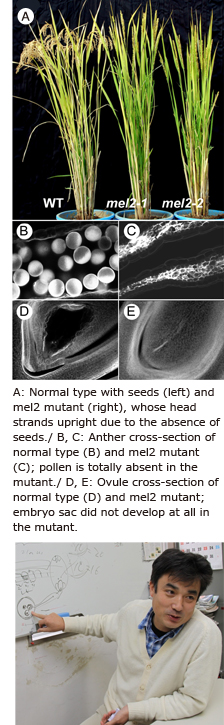
All rice mutants which I have identified are derived from “Rice Mutant Panel” programm developed by the National Institute of Agrobiological Sciences, Tsukuba, Japan. We selected mutant plants with no seed set and discovered the rice gene MEL2. We uncovered that this gene regulates transition from mitosis to meiosis during rice reproduction.
For an angiosperm to leave seeds, it needs a mechanism that produces male and female gametophytes (pollen and embryo sac) simultaneously at an appropriate season and realizes synchronous fertilization. Plants prepare many genetic systems for synchronous fertilization. Sensoring the change in environmental conditions such as day length and temperatures and initiating the transition to reproductive phase is one of important phenomena in the early part of this process. The establishment of synchrony of male meiosis, which is the main theme of my paper published in 2011, is known as another important system. Primordial germ cells, which are generated in large numbers in the anther primordium, repeat asynchronous premeiotic mitosis initially to increase the number of cells. Later, at a certain moment, germ cells in anther enter into meiosis at the same time. Pistil is also believed to have a mechanism that synchronizes meiosis with the male side. However, nothing was known about the molecular mechanism of a plant that supports transition to meiosis.
We found MEL2 gene expressed specifically in germ cells in anther and pistil. While MEL2 protein has three motifs, each of which is ubiquitously found in proteins of all organisms, the same motif combination with rice MEL2 is known to exist only in Gramineae or monocotyledonous plants. One of them is a RNA-recognition-motif (RRM), which is expected to bind with RNA. This RRM is very similar to human DAZAP1 in terms of sequence and motif. DAZAP1 is known to bind to DAZ, the protein responsible for human azoospermia. I think it is extremely intriguing that the gene implicated in plant meiosis resembles the gene associated with human azoospermia.
As a result of our study, we concluded that MEL2 is the master gene that regulates the timing of transition from pre-meiotic mitosis to meiosis. We believe that MEL2 regulates the cell division cycle of rice germ cells, specifically the beginning of DNA replication immediately before meiosis, and that this regulatory system is also used for the synchronization of meiosis in the anther. This seems to explain why MEL2 mutants can’t properly execute transition from mitosis to meiosis, resulting in loss of synchronicity and non-production of pollen.
What do you think was particularly well evaluated in your study?
There has been very active research in and outside Japan concerning “how flowers are made,” and a large number of related genes have already been identified. On the other hand, no systematic research had been carried out about the process from the birth of plant primordial germ cells to meiosis before ours. In view of this, I think our study was particularly appreciated in that it found a key to elucidating an unknown area and that it brought about the world’s first discovery and analysis of the plant gene required for transition to meiosis.
However, our study did not proceed very smoothly. We found MEL2 mutant itself in 2000, but it took us a full ten years to complete the paper. Since we had to follow the life of rice from the formation of germ cells to that of seeds, it required a great amount of time and patience. Moreover, we had the difficulty of having to analyze heterozygous mutants since MEL2 homozygous mutants produce no seeds. We also had to repeat trial and error before establishing methods for collecting and storing samples, that is, very young ears of rice, because it was an untrodden research area. At the same time, I am proud to say that our research had the originality of linking cellular-level phenomena in the early stage of reproduction with individual-level phenomena using mutant rice, thanks to all those difficulties.
Would it be possible to put the research achievements to practical application, for example in agriculture? What is your future goal?
They can’t be readily applied to agriculture, but I believe that there is a possibility of developing the research findings into measures against plant infertility caused by exceptionally cold weather and other environmental stresses. For example, damage to agriculture due to exceptionally cold weather on the Pacific side of the Tohoku region in Japan is known by the name Yamase. When Yamase happens, the cells that cover rice germ cells (tapetal cells) grow excessively large and die due to low temperatures; therefore, no meiosis, no pollen. Since the same phenomenon is found in MEL2 mutants, I believe it would be possible to discover why rice germ cells are vulnerable to exceptionally high or low temperatures and find solutions to mitigate damage by analyzing the disruption of progression into meiosis and tapetal cell enlargement.

Regulated transition to meiosis and synchronous male meiosis are commonly found in angiosperm species. In the future, I’d like to study what gene performs the function corresponding to that of rice’s MEL2 in dicotyledonous plants such as Arabidopsis thaliana. As well, I’d like to clarify as rapidly as possible the RNA sequence to which MEL2 RRM binds in order to discover what genetic mechanism MEL2 regulates, and how, by specifying the binding substrate of MEL2. I have identified and analyzed five rice genes related to reproduction including MEL2 so far. I’d also like to clarify how plant germ cells, characterized by totipotency and permanency, are generated within the context of plant life.
(Interviewed by Naoko Nishimura on Dec. 16, 2010)
A novel RNA-recognition-motif protein is required for premeiotic G1/S-phase transition in rice
(Oryza sativa L.)
Ken-Ichi Nonomura1,2,6, Mitsugu Eiguchi1, Mutsuko Nakano1, Kazuya Takashima1, Norio Komeda1,2, Satoshi Fukuchi2,3, Saori Miyazaki1,2, Akio Miyao4, Hirohiko Hirochika4, Nori Kurata2,5>
1. Experimental Farm, National Institute of Genetics>
2. Department of Genetics, School of Life Science, SOKENDAI>
3. Laboratory for DNA Data Analysis, National Institute of Genetics>
4. Division of Genome and Biodiversity Research, National Institute of Agrobiological Sciences>
5. Plant Genetics Laboratory, National Institute of Genetics>
PLoS Genetics, 7(1): e1001265. DOI:10.1371/journal.pgen.1001265















