Efficient production of recombinant multi-subunit proteins using the Tol2 transposon
Kawakami Group / Laboratory of Molecular and Developmental Biology
Production of multi-subunit proteins in CHO cells by transposase-mediated integration of subunit-splitting vectors.
Keina Yamaguchi, Risa Ogawa, Masayoshi Tsukahara and Koichi Kawakami
Scientific reports (2025) 15, 18512 DOI:10.1038/s41598-025-03301-3
Chinese Hamster Ovary (CHO) cells are widely used for therapeutic protein production. We previously developed a method using the Tol2 transposon system to efficiently establish protein-producing cell lines via multiple genomic integrations of the gene of interest. In this study, we introduced two separate vectors—one carrying the light chain (LC) gene and the other the heavy chain (HC) gene—into CHO cells, rather than a single vector with both genes. As a result, we obtained cell lines that stably produced monoclonal antibodies for up to 12 weeks and showed high productivity in fed-batch culture. Each cell line exhibited variable copy numbers of integrated LC and HC vectors, depending on the antibody type. High-producing lines had optimal ratios of LC and HC gene copies. Notably, optimization was achieved even when the drug resistance gene was present only in the HC vector. These findings highlight the potential of the Tol2 system for efficient production of monoclonal antibodies and other multi-subunit proteins.
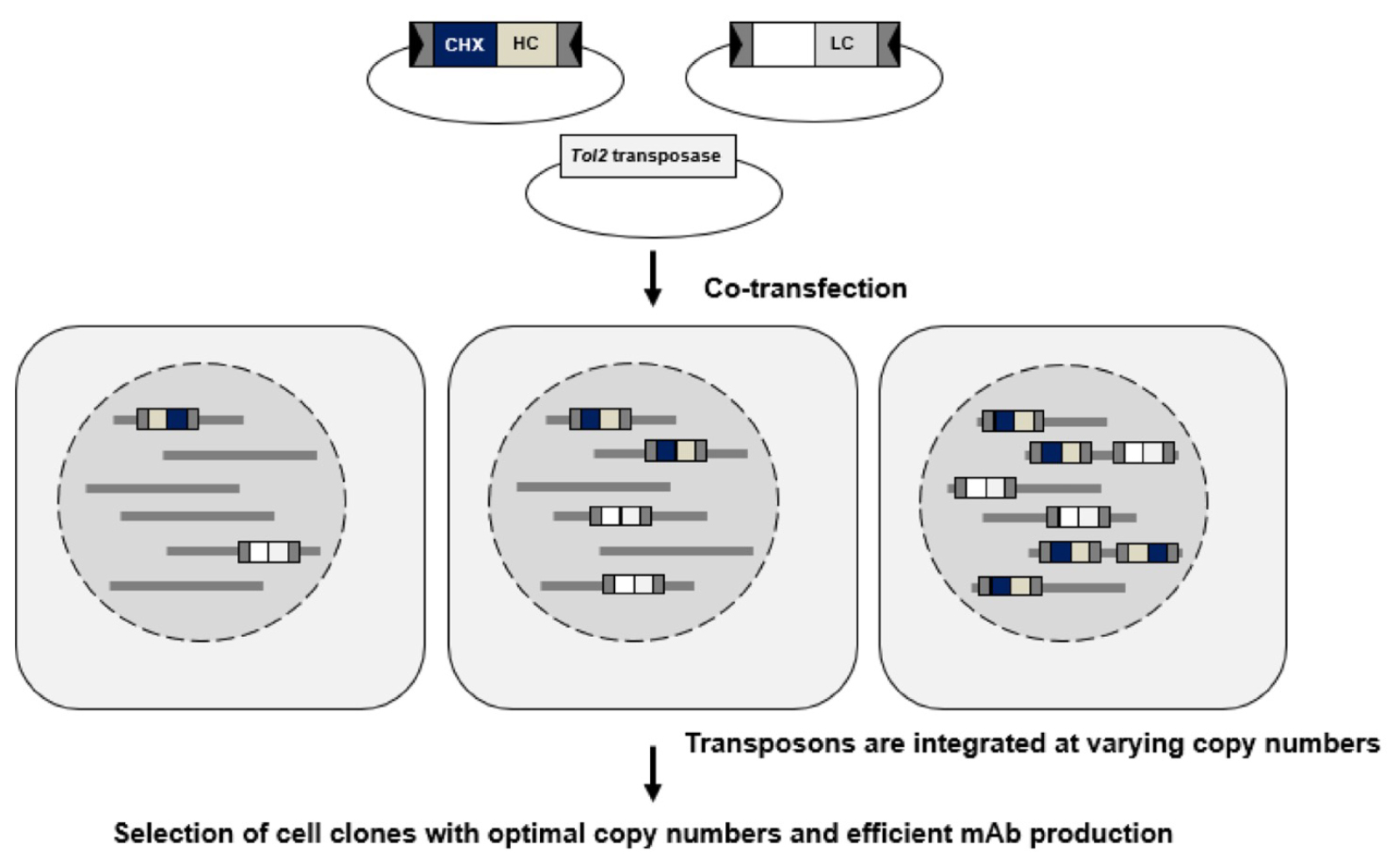
Figure: Overview of the method.
Separate transposon vectors carrying the heavy chain (HC) and light chain (LC) genes are co-transfected into CHO cells. The drug resistance gene (cycloheximide resistance) is present only in the LC vector. In the transfected cells, the HC and LC vectors are integrated into the host genome in varying copy numbers. Cell lines carrying optimal copy numbers of both HC and LC vectors can be selected as cell lines that efficiently produce monoclonal antibodies.















