The First Dimerization-Dependent Protein Knockdown Method in Photosynthetic Organisms
Miyagishima Group / Symbiosis and Cell Evolution Laboratory
Development of a rapamycin-inducible protein-knockdown system in the unicellular red alga Cyanidioschyzon merolae.
Takayuki Fujiwara, Shunsuke Hirooka, Shota Yamashita, Fumi Yagisawa and Shin-ya Miyagishima
Plant Physiology (2024) kiae316 DOI:10.1093/plphys/kiae316
An inducible protein-knockdown system is highly effective for investigating the functions of proteins and mechanisms essential for the survival and growth of organisms. However, this technique is not available in photosynthetic eukaryotes. The unicellular red alga Cyanidioschyzon merolae possesses a very simple cellular and genomic architecture and is genetically tractable but lacks RNA interference machinery. In this study, we developed a protein-knockdown system in this alga. The constitutive system utilizes the destabilizing activity of the FK506-binding protein 12 (FKBP12)-rapamycin-binding (FRB) domain of human target of rapamycin kinase or its derivatives to knock down target proteins. In the inducible system, rapamycin treatment induces the heterodimerization of the human FRB domain fused to the target proteins with the human FKBP fused to S-phase kinase associated protein 1 or Cullin 1, subunits of the SCF E3 ubiquitin ligase. This results in the rapid degradation of the target proteins through the ubiquitin-proteasome pathway. With this system, we successfully degraded endogenous essential proteins such as the chloroplast division protein dynamin-related protein 5B and E2 transcription factor, a regulator of the G1/S transition, within 2 to 3 h after rapamycin administration, enabling the assessment of resulting phenotypes. This rapamycin-inducible protein-knockdown system contributes to the functional analysis of genes whose disruption leads to lethality.
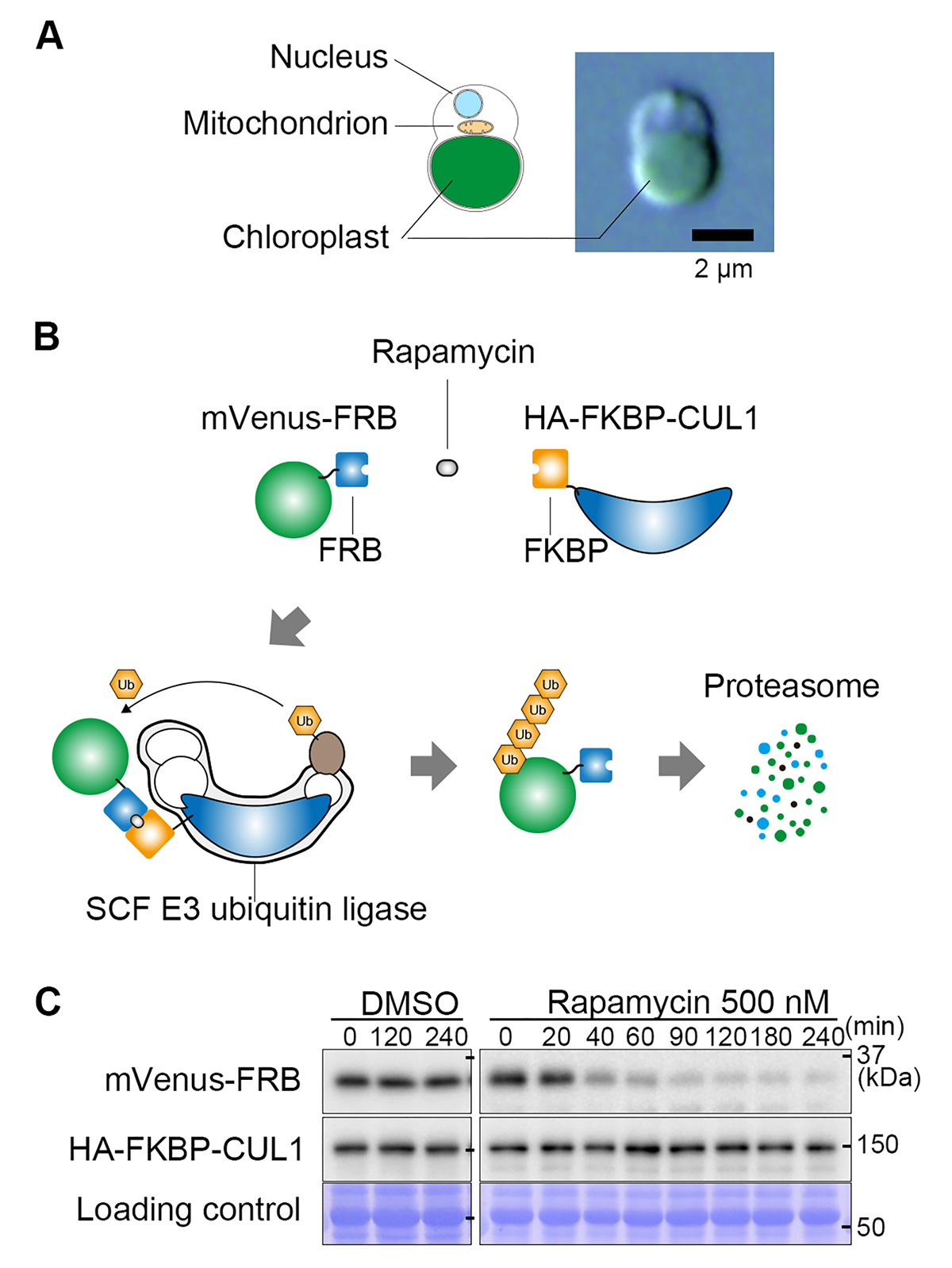
Figure: A rapamycin-inducible protein knockdown system.
(A) The cellular structure of the unicellular red alga Cyanidioschyzon merolae and a differential interference contrast microscopy image.
(B) Mechanism of the rapamycin-inducible protein knockdown system. FRB tags and FKBP proteins form a heterodimer in the presence of rapamycin. The target protein fused with an FRB tag (in this figure, the fluorescent protein mVenus) and the CUL1 subunit of ubiquitin ligase fused with FKBP bind in the presence of rapamycin, resulting in the ubiquitination of FRB-mVenus. The ubiquitinated FRB-mVenus is rapidly degraded by the proteasome.
(C) Immunoblot analysis showing the decrease in mVenus-FRB protein level after the addition of rapamycin.















