Efficient production of recombinant proteins using the Tol2 transposon
Efficient production of recombinant proteins in suspension CHO cells culture using the Tol2 transposon system coupled with cycloheximide resistance selection.
Keina Yamaguchi, Risa Ogawa, Masayoshi Tsukahara and Koichi Kawakami Scientific reports (2023) 13, 7628 DOI:10.1038/s41598-023-34636-4DNA recombination techniques in mammalian cells has been applied to the production of therapeutic proteins for several decades. To be used for commercial production, established cell lines should stably express target proteins with high productivity and acceptable quality for human use. In the conventional transfection method, the screening process is laborious and time-consuming since superior cell lines had to be selected from an enormous number of transfected cell pools and clonal cell lines with a wide variety of transgene insertion locations. In this study, we demonstrated that the combination of a Tol2 transposon system and cell selection by cycloheximide resistance is an efficient method to express therapeutic proteins, such as human antibody in suspension culture of Chinese hamster ovary cells. The resulting stable cell lines showed constant productivity and cell growth over long enough cultivation periods for recombinant protein production. We anticipate that this approach will prove widely applicable to protein production in research and development of pharmaceutical products.
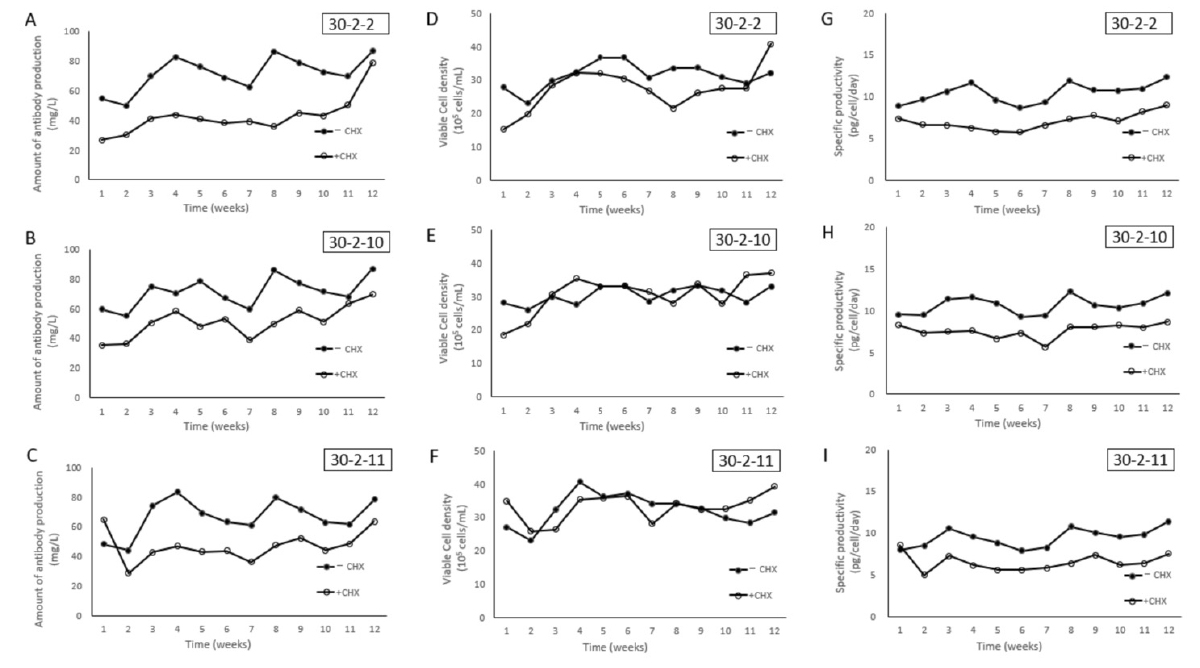
Figure: Antibody productivity and cell growth of clonal cell lines 30-2-2, 30-2-10, 30-2-11 in the presence or absence of CHX selection. Antibody productivity (A, B and C), cell growth (D, E and F) and specific antibody productivity (pg/cell/day) (G, H and I) in 30-2-2, 30-2-10, 30-2-11 were measured in the presence (open circles) or absence (black circles) of CHX selection pressure.















