Archive
- Home
- February 2026
- January 2026
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- July 2006
- June 2006
- May 2006
- April 2006
- January 2006
- June 2005
- May 2005
- April 2005
- February 2005
- December 2004
- November 2004
- October 2004
- August 2004
- July 2004
- June 2004
- May 2004
- February 2004
- January 2004
- December 2003
- August 2003
- July 2003
- June 2003
- May 2003
- March 2003
- January 2003
- December 2002
- October 2002
- May 2002
- April 2002
- February 2002
- January 2002
- August 2001
- May 2001
- April 2001
- February 2001
- August 2000
- July 2000
Morphogenesis of bacterial cells analyzed by a high performance sequencer
Microbial Genetics Laboratory・Niki Group
Comparative Genomics Laboratory・Fujiyama Group
Genome Biology Laboratory・Kohara Group
Wild-type E. coli are rod shape (Figure A, left). To make rod shaped cells, it is necessary that many proteins form complexes and function properly. E. coli cells are surrounded by rigid peptidoglycan (PG) layer and have to synthesize PG correctly. So far, we have identified RodZ required for determination of cell shape.
RodZ-deficient mutant is round shape (Figure A, middle) and grows slower than wild-type E. coli cell. To reveal function of RodZ protein, we isolated suppressor mutants that restore cell growth and shape of the rodZ mutant. To map the mutation sites in the suppressor mutants, we sequenced whole genome of twenty-nine mutants by a next-generation sequencer Solexa. This is the first report that mutation sites in ~30 mutants are determined by whole genome sequencing.
Most of the mutations were found in mreB, mrdA, or mrdB genes. It has been hypothesized that MreB, PBP2 encoded by mrdA gene, and RodA encoded by mrdB gene function with RodZ. Especially, twenty of twenty-nine mutants had a mutation in mreB gene. In addition, these mutations were clustered in domain 1A, one of the subdomain of MreB protein. These mutations change properties of MreB protein so that E. coli can form rod shape without RodZ protein. We also found that mutants of PBP2 and RodA change properties of MreB. Thus, we concluded that RodZ regulates function of MreB to form rod shape of E. coli.
This work was done by a collaboration of Niki lab, Fujiyama lab, and Kohara lab.
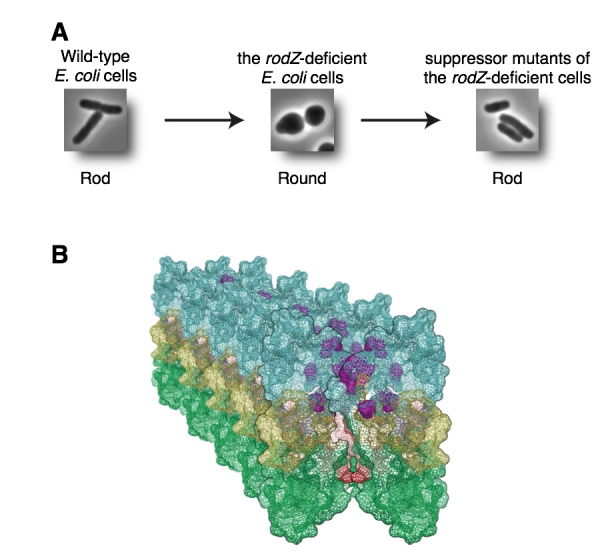
(A) Wild-type E. coli is rod (left). RodZ-deficient E. coli cell is round (middle). Suppressor mutants are rod (right). (B) Mutation sites shown by purple are clustered in domain 1A and it is a surface between MreB filaments. These mutations change properties of MreB filaments.
Structural core for kinetochore formation
Division of Molecular Genetics・Fukagawa Group
The kinetochore forms a dynamic interface with microtubules from the mitotic spindle during mitosis. The Ndc80 complex acts as the key microtubule-binding complex at kinetochores. However, it is unclear how the Ndc80 complex associates with the inner kinetochore proteins that assemble upon centromeric chromatin. Here, based on a high-resolution structural analysis, we demonstrate that the N-terminal region of vertebrate CENP-T interacts with the “RWD” domain in the Spc24/25 portion of the Ndc80 complex. Phosphorylation of CENP-T strengthens a cryptic hydrophobic interaction between CENP-T and Spc25 resulting in a phospho-regulated interaction that occurs without direct recognition of the phosphorylated residue. The Ndc80 complex interacts with both CENP-T and the Mis12 complex, but we find that these interactions are mutually exclusive, supporting a model in which two distinct pathways target the Ndc80 complex to kinetochores. Our results provide a model for how the multiple protein complexes at kinetochores associate in a phospho-regulated manner.
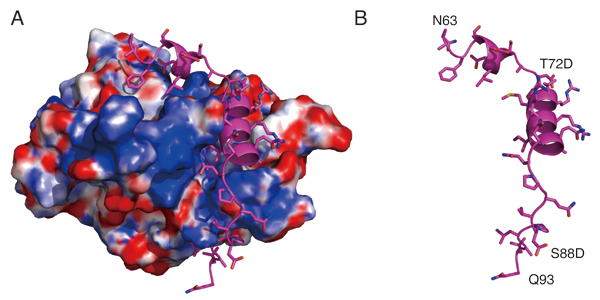
Structural model showing the surface charge of the Spc24/25 complex interacting with phospho-mimetic CENP-T peptide (Cyan). (B) Structural model showing the phospho-mimetic CENP-T peptide from the CENP-T-Spc24/25 complex structure in (A) on its own.
Takehiko Kobayashi, Professor of Cytogenetics, was awarded “the 29th Inoue prize” by Inoue Foundation for Science.

 Title
Study on gene amplification and its relationship to cancer and aging
Takehiko Kobayashi, Professor of Cytogenetics, was awarded “the 29th Inoue prize” by Inoue Foundation for Science. This prize is awarded to scientists who conducted basic and original scientific works. Takehiko Kobayashi revealed the molecular mechanism of “gene amplification” that contributed to cancer and aging.
Inoue Foundation for Science
※This site is presented only in Japanese.
Title
Study on gene amplification and its relationship to cancer and aging
Takehiko Kobayashi, Professor of Cytogenetics, was awarded “the 29th Inoue prize” by Inoue Foundation for Science. This prize is awarded to scientists who conducted basic and original scientific works. Takehiko Kobayashi revealed the molecular mechanism of “gene amplification” that contributed to cancer and aging.
Inoue Foundation for Science
※This site is presented only in Japanese.Identification of male promoting signal in mouse germ cells
Mammalian Development Laboratory・Saga Group
In the mouse, testicular development is triggered in somatic cells by the function of Sry followed by the activation of fibroblast growth factor (FGF) 9, which regulates testicular differentiation in both somatic and germ cells. However, the mechanism is unknown. We show here that Nodal/Activin signaling pathway is activated in both male gem cells and somatic cells. The disruption of Nodal/Activin signaling drives male germ cells into meiosis and causes ectopic initiation of female-specific genes in somatic cells. Furthermore, we prove that Nodal/Activin-A works directly on male germ cells to induce male specific gene, Nanos2 independently of FGF9. We conclude that Nodal/Activin signaling is required for testicular development and propose a model in which Nodal/Activin-A acts downstream of FGF signaling to promote male germ cell fate and protect somatic cells from initiating female differentiation.
This study is conducted by Quan Wu who is a current student of SOKENDAI.
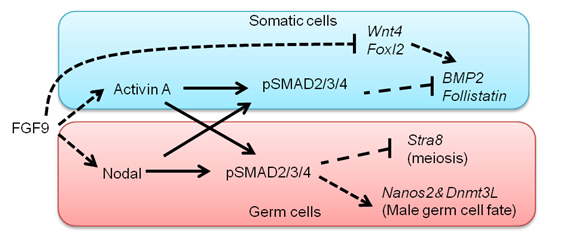
A model proposed by our study. FGF signals activate Nodal/Activin signaling pathway in both somatic cells and germ cells. Nodal/Activin-A triggers male sex differentiation by inducing male-specific genes, Nanos2 and Dnmt3L. Meanwhile, it suppresses Stra8 that is an essential gatekeeper of meiosis. In somatic cells Nodal/Activin-A thwarts the process of female differentiation by inhibiting Bmp2 and Follistatin.















