A mechanism of cell fate maintenance through histone acetylation
Multicellular Organization Laboratory • Sawa Group
HTZ-1/H2A.z and MYS-1/MYST HAT act redundantly to maintain cell fates in somatic gonadal cells through repression of ceh-22 in C. elegans
Yukimasa Shibata, Hitoshi Sawa and Kiyoji Nishiwaki
Development 141, 209-218 (2013), doi:10.1242/dev.090746
The stable maintenance of cell fates is essential for proper development and tissue homeostasis. Abnormalities in the maintenance lead to cancer or loss of important cell types. We have previously shown histone acetyltransferases (MYS-1 and MYS-2) and an acetylated-histone binding protein (BET-1) are required for the maintenance of cell fates in C. elegans, indicating that histone acetylation plays important roles in the process. It is not known, however, how BET-1 maintains cell fates.
It is known, in yeast, that BDF1 (an homolog of BET-1) forms a complex with a chromatin-remodeling factor SWR1 and regulates the deposition of histone H2A variant HTZ1/H2A.z. We have shown that SSL-1/SWR1 and HTZ-1/H2A.z are required for the cell fate maintenance in C. elegans. These proteins suppress the ectopic production of germline niche cells (DTC) by maintaining fates of somatic cells in the gonad. We found that the ceh-22 gene that encodes a transcription factor required for the DTC production is a direct target of HTZ-1 and that ceh-22 transcription is repressed by BET-1, MYS-1 and HTZ-1. Although it is well known that histone acetylation is involved in transcriptional activation, our results indicate that it also represses transcription.
This study has been carried out as collaboration with Drs. Shibata and Nishiwaki at Kwansei Gakuin University.
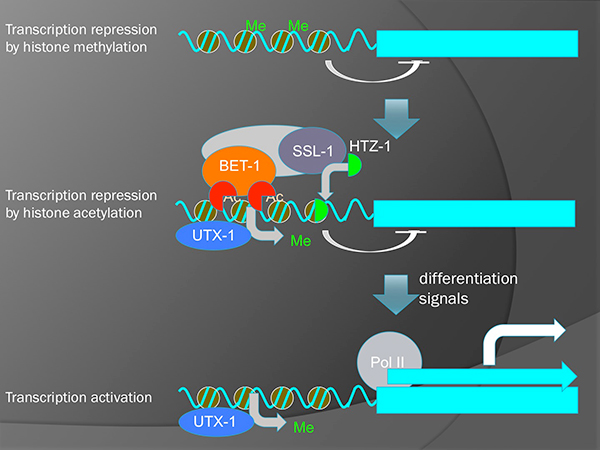
A model for two-step activation of transcription. Removal of H3K27 methylation by UTX-1 potentiates genes to be transcribed. However, actual transcription is still inhibited by BET-1 and HTZ-1 without the reception of differentiation signals.















